A Photo-clickable ATP-Mimetic Reveals Nucleotide Interactors in the Membrane Proteome
- PMID: 32521230
- PMCID: PMC7552919
- DOI: 10.1016/j.chembiol.2020.05.010
A Photo-clickable ATP-Mimetic Reveals Nucleotide Interactors in the Membrane Proteome
Abstract
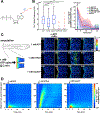
ATP is an important energy metabolite and allosteric signal in health and disease. ATP-interacting proteins, such as P2 receptors, control inflammation, cell death, migration, and wound healing. However, identification of allosteric ATP sites remains challenging, and our current inventory of ATP-controlled pathways is likely incomplete. Here, we develop and verify mipATP as a minimally invasive photoaffinity probe for ATP-interacting proteins. Its N6 functionalization allows target enrichment by UV crosslinking and conjugation to reporter tags by "click" chemistry. The additions are compact, allowing mipATP to completely retain the calcium signaling responses of native ATP in vitro and in vivo. mipATP specifically enriched for known nucleotide binders in A549 cell lysates and membrane fractions. In addition, it retrieved unannotated ATP interactors, such as the FAS receptor, CD44, and various SLC transporters. Thus, mipATP is a promising tool to identify allosteric ATP sites in the proteome.
Keywords: ATP; ATP photoaffinity probe; calcium signaling; chemical proteomics; photoaffinity labeling; purinergic signaling; quantitative proteomics; target discovery.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



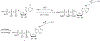
References
-
- Adachi J, Kishida M, Watanabe S, Hashimoto Y, Fukamizu K, and Tomonaga T (2014). Proteome-wide discovery of unknown ATP-binding proteins and kinase inhibitor target proteins using an ATP probe. J. Proteome Res 13, 5461–5470. - PubMed
-
- Arguello AE, DeLiberto AN, and Kleiner RE (2017). RNA Chemical Proteomics Reveals the N6-Methyladenosine (m6A)-Regulated Protein-RNA Interactome. J. Am. Chem. Soc 139, 17249–17252. - PubMed
-
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, and Seed B (1990). CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303–1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

