Variable Outcomes in Neural Differentiation of Human PSCs Arise from Intrinsic Differences in Developmental Signaling Pathways
- PMID: 32521257
- PMCID: PMC7296348
- DOI: 10.1016/j.celrep.2020.107732
Variable Outcomes in Neural Differentiation of Human PSCs Arise from Intrinsic Differences in Developmental Signaling Pathways
Abstract
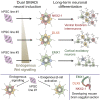
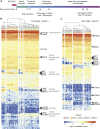
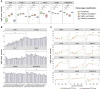
Directed differentiation of human pluripotent stem cells varies in specificity and efficiency. Stochastic, genetic, intracellular, and environmental factors affect maintenance of pluripotency and differentiation into early embryonic lineages. However, factors affecting variation in in vitro differentiation to defined cell types are not well understood. To address this, we focused on a well-established differentiation process to cerebral cortex neural progenitor cells and their neuronal progeny from human pluripotent stem cells. Analysis of 162 differentiation outcomes of 61 stem cell lines derived from 37 individuals showed that most variation occurs along gene expression axes reflecting dorsoventral and rostrocaudal spatial expression during in vivo brain development. Line-independent and line-dependent variations occur, with the latter driven largely by differences in endogenous Wnt signaling activity. Tuning Wnt signaling during a specific phase early in the differentiation process reduces variability, demonstrating that cell-line/genome-specific differentiation outcome biases can be corrected by controlling extracellular signaling.
Keywords: Hh signalling; Wnt signalling; cortical differentiation; dual SMAD inhibition; hPSC variation; human puripotent stem cell; neural induction; patterning of the cortex; regional identity specification; transcriptional profiling.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





References
-
- Backman M., Machon O., Mygland L., van den Bout C.J., Zhong W., Taketo M.M., Krauss S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev. Biol. 2005;279:155–168. - PubMed
-
- Bar-Nur O., Russ H.A., Efrat S., Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. - PubMed
-
- Bauwens C.L., Peerani R., Niebruegge S., Woodhouse K.A., Kumacheva E., Husain M., Zandstra P.W. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. - PubMed
-
- Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B. 1995;57:289–300.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

