Pathogenesis and Clinical Management of Mesenteric Fibrosis in Small Intestinal Neuroendocine Neoplasms: A Systematic Review
- PMID: 32521677
- PMCID: PMC7357094
- DOI: 10.3390/jcm9061777
Pathogenesis and Clinical Management of Mesenteric Fibrosis in Small Intestinal Neuroendocine Neoplasms: A Systematic Review
Abstract
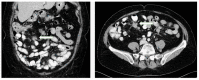
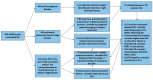
Mesenteric fibrosis (MF) constitutes an underrecognized sequela in patients with small intestinal neuroendocrine neoplasms (SI-NENs), often complicating the disease clinical course. The aim of the present systematic review, carried out by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology, is to provide an update in evolving aspects of MF pathogenesis and its clinical management in SI-NENs. Complex and dynamic interactions are present in the microenvironment of tumor deposits in the mesentery. Serotonin, as well as the signaling pathways of certain growth factors play a pivotal, yet not fully elucidated role in the pathogenesis of MF. Clinically, MF often results in significant morbidity by causing either acute complications, such as intestinal obstruction and/or acute ischemia or more chronic conditions involving abdominal pain, venous stasis, malabsorption and malnutrition. Surgical resection in patients with locoregional disease only or symptomatic distant stage disease, as well as palliative minimally invasive interventions in advanced inoperable cases seem clinically meaningful, whereas currently available systemic and/or targeted treatments do not unequivocally affect the development of MF in SI-NENs. Increased awareness and improved understanding of the molecular pathogenesis of MF in SI-NENs may provide better diagnostic and predictive tools for its timely recognition and intervention and also facilitates the development of agents targeting MF.
Keywords: CTGF; FGF; PDGF; TGF; VEGF; mesenteric fibrosis; neuroendocrine tumors; serotonin; small intestine.
Conflict of interest statement
The authors declare that there is no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

