DHA Modulates Immune Response and Mitochondrial Function of Atlantic Salmon Adipocytes after LPS Treatment
- PMID: 32521827
- PMCID: PMC7312884
- DOI: 10.3390/ijms21114101
DHA Modulates Immune Response and Mitochondrial Function of Atlantic Salmon Adipocytes after LPS Treatment
Abstract
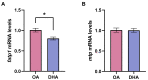
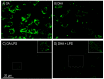
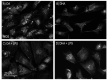
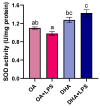
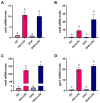
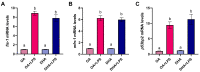
Adipocytes play a central role in overall energy homeostasis and are important contributors to the immune system. Fatty acids (FAs) act as signaling molecules capable to modulate adipocyte metabolism and functions. To identify the effects of two commonly used FAs in Atlantic salmon diets, primary adipocytes were cultured in the presence of oleic (OA) or docosahexaenoic (DHA) acid. DHA decreased adipocyte lipid droplet number and area compared to OA. The increase in lipid load in OA treated adipocytes was paralleled by an increase in iNOS activity and mitochondrial SOD2-GFP activity, which was probably directed to counteract increase in oxidative stress. Under lipopolysaccharide (LPS)-induced inflammation, DHA had a greater anti-inflammatory effect than OA, as evidenced by the higher SOD2 activity and the transcriptional regulation of antioxidant enzymes and pro- and anti-inflammatory markers. In addition, DHA maintained a healthy mitochondrial structure under induced inflammation while OA led to elongated mitochondria with a thin thread like structures in adipocytes exposed to LPS. Overall, DHA possess anti-inflammatory properties and protects Atlantic salmon against oxidative stress and limits lipid deposition. Furthermore, DHA plays a key role in protecting mitochondria shape and function.
Keywords: Salmo salar; adipocytes; antioxidant enzymes; mitochondria; n-3 HUFAs; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Aas T.S., Ytrestøyl T., Åsgård T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2016. Aquac. Rep. 2019;15:100216. doi: 10.1016/j.aqrep.2019.100216. - DOI
-
- Colombo S.M., Foroutani M.B., Parrish C.C. Fats and Oils in Aquafeed Formulations. In: Shahidi F., editor. Bailey’s Industrial Oil and Fat Products. Wiley Online Library; Hoboken, NJ, USA: 2020. pp. 1–28.
-
- Todorčević M., Kjær M.A., Djaković N., Vegusdal A., Torstensen B.E., Ruyter B. N-3 HUFAs affect fat deposition, susceptibility to oxidative stress, and apoptosis in Atlantic salmon visceral adipose tissue. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009;152:135–143. doi: 10.1016/j.cbpb.2008.10.009. - DOI - PubMed
-
- Weil C., Lefèvre F., Bugeon J. Characteristics and metabolism of different adipose tissues in fish. Rev. Fish Biol. Fish. 2013;23:157–173. doi: 10.1007/s11160-012-9288-0. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

