ICT-based adherence monitoring in kidney transplant recipients: a randomized controlled trial
- PMID: 32522263
- PMCID: PMC7285710
- DOI: 10.1186/s12911-020-01146-6
ICT-based adherence monitoring in kidney transplant recipients: a randomized controlled trial
Abstract
Background: Prior studies have explored the use of regular reminders to improve adherence among kidney transplant recipients (KTRs), but none have included real-time alarms about drug dosage, frequency, and interval. In the present study, we aimed to evaluate the efficacy and stability of an information and communication technology (ICT)-based centralized monitoring system for increasing medication adherence among Korean KTRs.
Methods: In this prospective, multicenter, randomized controlled study, enrolled KTRs were randomized to either the ICT-based centralized monitoring group or control group. The ICT-based centralized monitoring system alerted both patients and medical staff with texts and pill box alarms if there was a missed dose or a dosage/time error. We compared the two groups in terms of medication adherence and transplant outcomes over 6 months, and evaluated patient satisfaction with the ICT-based monitoring system.
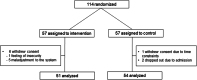
Results: Among 114 enrolled KTRs, 57 were assigned to the ICT-based centralized monitoring group and 57 to the control group. The two groups did not significantly differ in mean adherence at each follow-up visit. The intrapatient variability of tacrolimus and mycophenolic acid levels, renal function, and adverse transplant outcomes did not differ between the intervention and control groups, or between the intervention group with feedback generation and the intervention group without feedback generation. Patients showed high overall satisfaction with the ICT-based centralized monitoring system, which significantly improved across the study period (p = 0.012).
Conclusions: Due to high baseline adherence, the ICT-based centralized monitoring system did not maximize medication adherence or enhance transplant outcomes among Korean KTRs. However, patients were highly satisfied with the system. Our results suggest that the ICT-based centralized monitoring system could be successfully applied in clinical trials.
Trial registration: ClinicalTrials.gov, NCT03136588. Registered 20 April 2017 - Retrospectively registered.
Keywords: Adherence; Feedback; Information and communication technology; Kidney transplantation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–399. doi: 10.1111/j.1600-6143.2011.03840.x. - DOI - PubMed
-
- Weng FL, Chandwani S, Kurtyka KM, Zacker C, Chisholm-Burns MA, Demissie K. Prevalence and correlates of medication non-adherence among kidney transplant recipients more than 6 months post-transplant: a cross-sectional study. BMC Nephrol. 2013;14:261. doi: 10.1186/1471-2369-14-261. - DOI - PMC - PubMed
-
- Joost R, Dorje F, Schwitulla J, Eckardt KU, Hugo C. Intensified pharmaceutical care is improving immunosuppressive medication adherence in kidney transplant recipients during the first post-transplant year: a quasi-experimental study. Nephrol Dial Transpl. 2014;29(8):1597–1607. doi: 10.1093/ndt/gfu207. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

