Intestinal microbiota: a new force in cancer immunotherapy
- PMID: 32522267
- PMCID: PMC7288675
- DOI: 10.1186/s12964-020-00599-6
Intestinal microbiota: a new force in cancer immunotherapy
Abstract
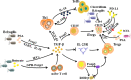
Cancer displays high levels of heterogeneity and mutation potential, and curing cancer remains a challenge that clinicians and researchers are eager to overcome. In recent years, the emergence of cancer immunotherapy has brought hope to many patients with cancer. Cancer immunotherapy reactivates the immune function of immune cells by blocking immune checkpoints, thereby restoring the anti-tumor activity of immune cells. However, immune-related adverse events are a common complication of checkpoint blockade, which might be caused by the physiological role of checkpoint pathways in regulating adaptive immunity and preventing autoimmunity. In this context, the intestinal microbiota has shown great potential in the immunotherapy of cancer. The intestinal microbiota not only regulates the immune function of the body, but also optimizes the therapeutic effect of immune checkpoint inhibitors, thus reducing the occurrence of complications. Therefore, manipulating the intestinal microbiota is expected to enhance the effectiveness of immune checkpoint inhibitors and reduce adverse reactions, which will lead to new breakthroughs in immunotherapy and cancer management. Video abstract.
Keywords: CTLA-4; Cancer immunotherapy; Checkpoint; FMT; ICIs; Microbiota; PD-1; PD-L1.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Ge Z, Wu S, Zhang Z, Ding S. Mechanism of tumor cells escaping from immune surveillance of NK cells. Immunopharmacol Immunotoxicol. 2020:1–12. - PubMed
-
- Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, Sahebkar A. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234:16824–16837. - PubMed
-
- King-Kallimanis BL, Howie LJ, Roydhouse JK, Singh H, Theoret MR, Blumenthal GM, Kluetz PG. Patient reported outcomes in anti-PD-1/PD-L1 inhibitor immunotherapy registration trials: FDA analysis of data submitted and future directions. Clin Trials. 2019;16:322–326. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

