Notch-Mediated Generation of Monocyte-Derived Langerhans Cells: Phenotype and Function
- PMID: 32522485
- PMCID: PMC7758629
- DOI: 10.1016/j.jid.2020.05.098
Notch-Mediated Generation of Monocyte-Derived Langerhans Cells: Phenotype and Function
Abstract
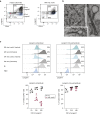
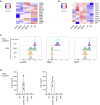
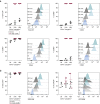
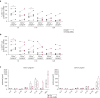
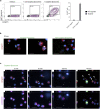
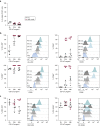
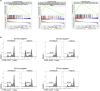
Langerhans cells (LCs) in the skin are a first line of defense against pathogens but also play an essential role in skin homeostasis. Their exclusive expression of the C-type lectin receptor Langerin makes them prominent candidates for immunotherapy. For vaccine testing, an easily accessible cell platform would be desirable as an alternative to the time-consuming purification of LCs from human skin. Here, we present such a model and demonstrate that monocytes in the presence of GM-CSF, TGF-β1, and the Notch ligand DLL4 differentiate within 3 days into CD1a+Langerin+cells containing Birbeck granules. RNA sequencing of these monocyte-derived LCs (moLCs) confirmed gene expression of LC-related molecules, pattern recognition receptors, and enhanced expression of genes involved in the antigen-presenting machinery. On the protein level, moLCs showed low expression of costimulatory molecules but prominent expression of C-type lectin receptors. MoLCs can be matured, secrete IL-12p70 and TNF-α, and stimulate proliferation and cytokine production in allogeneic CD4+ and CD8+ T cells. In regard to vaccine testing, a recently characterized glycomimetic Langerin ligand conjugated to liposomes demonstrated specific and fast internalization into moLCs. Hence, these short-term in vitro‒generated moLCs represent an interesting tool to screen LC-based vaccines in the future.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Braathen L.R., Thorsby E. Studies on human epidermal Langerhans cells. I. allo-activating and antigen-presenting capacity. Scand J Immunol. 1980;11:401–408. - PubMed
-
- Carpentier S., Vu Manh T.P., Chelbi R., Henri S., Malissen B., Haniffa M. Comparative genomics analysis of mononuclear phagocyte subsets confirms homology between lymphoid tissue-resident and dermal XCR1(+) DCs in mouse and human and distinguishes them from Langerhans cells. J Immunol Methods. 2016;432:35–49. - PMC - PubMed
Supplementary References
-
- Durinck S., Moreau Y., Kasprzyk A., Davis S., De Moor B., Brazma A. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics. 2005;21:3439–3440. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

