Persistent Zika Virus Clinical Susceptibility despite Reduced Viral Burden in Mice with Expanded Virus-Specific CD8+ T Cells Primed by Recombinant Listeria monocytogenes
- PMID: 32522837
- PMCID: PMC7343594
- DOI: 10.4049/jimmunol.1901412
Persistent Zika Virus Clinical Susceptibility despite Reduced Viral Burden in Mice with Expanded Virus-Specific CD8+ T Cells Primed by Recombinant Listeria monocytogenes
Abstract
Vaccines against Zika virus (ZIKV) infection that target CD8+ T cells are of considerable interest because Abs may enhance infection susceptibility. However, whether CD8+ T cells are protective or promote susceptibility to clinical infection symptoms remains uncertain. To more precisely investigate ZIKV-specific CD8+ T cells in isolation, we engineered a Listeria monocytogenes-based vector to express a single MHC class I-restricted immune dominant peptide, E294-302, from ZIKV envelope protein. We show accumulation of activated ZIKV-specific CD8+ T cells primed by recombinant L. monocytogenes is associated with reductions in circulating virus levels after ZIKV challenge in type I IFN receptor-deficient mice and wildtype mice administered neutralizing Abs against type I IFN receptor. Interestingly, susceptibility to ZIKV clinical infection including weight loss and mortality each persists and is neither significantly improved nor worsened compared with isogenic L. monocytogenes-primed control mice. These data demonstrating persistent ZIKV clinical susceptibility despite reduced viral burden in mice with expanded virus-specific CD8+ T cells highlights the need for targeting other adaptive immune components in developing vaccines against ZIKV infection.
Copyright © 2020 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest
Figures
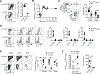
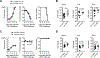
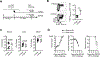

References
-
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, and Screaton GR. 2016. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 17: 1102–1108. - PMC - PubMed
-
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, and Corti D. 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353: 823–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

