ORF4 of the Temperate Archaeal Virus SNJ1 Governs the Lysis-Lysogeny Switch and Superinfection Immunity
- PMID: 32522850
- PMCID: PMC7394903
- DOI: 10.1128/JVI.00841-20
ORF4 of the Temperate Archaeal Virus SNJ1 Governs the Lysis-Lysogeny Switch and Superinfection Immunity
Abstract
Recent environmental and metagenomic studies have considerably increased the repertoire of archaeal viruses and suggested that they play important roles in nutrient cycling in the biosphere. However, very little is known about how they regulate their life cycles and interact with their hosts. Here, we report that the life cycle of the temperate haloarchaeal virus SNJ1 is controlled by the product ORF4, a small protein belonging to the antitoxin MazE superfamily. We show that ORF4 controls the lysis-lysogeny switch of SNJ1 and mediates superinfection immunity by repression of genomic DNA replication of the superinfecting viruses. Bioinformatic analysis shows that ORF4 is highly conserved in two SNJ1-like proviruses, suggesting that the mechanisms for lysis-lysogeny switch and superinfection immunity are conserved in this group of viruses. As the lysis-lysogeny switch and superinfection immunity of archaeal viruses have been poorly studied, we suggest that SNJ1 could serve as a model system to study these processes.IMPORTANCE Archaeal viruses are important parts of the virosphere. Understanding how they regulate their life cycles and interact with host cells provide crucial insights into their biological functions and the evolutionary histories of viruses. However, mechanistic studies of the life cycle of archaeal viruses are scarce due to a lack of genetic tools and demanding cultivation conditions. Here, we discover that the temperate haloarchaeal virus SNJ1, which infects Natrinema sp. strain J7, employs a lysis-lysogeny switch and establishes superinfection immunity like bacteriophages. We show that its ORF4 is critical for both processes and acts as a repressor of the replication of SNJ1. These results establish ORF4 as a master regulator of SNJ1 life cycle and provides novel insights on the regulation of life cycles by temperate archaeal viruses and on their interactions with host cells.
Keywords: Archaea; SNJ1; lysis-lysogeny switch; superinfection immunity; temperate virus.
Copyright © 2020 American Society for Microbiology.
Figures
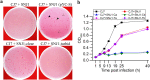
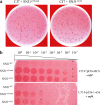
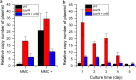



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

