The architecture of the Gram-positive bacterial cell wall
- PMID: 32523118
- PMCID: PMC7308169
- DOI: 10.1038/s41586-020-2236-6
The architecture of the Gram-positive bacterial cell wall
Abstract
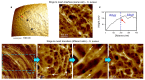
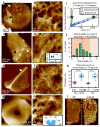
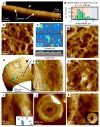
The primary structural component of the bacterial cell wall is peptidoglycan, which is essential for viability and the synthesis of which is the target for crucial antibiotics1,2. Peptidoglycan is a single macromolecule made of glycan chains crosslinked by peptide side branches that surrounds the cell, acting as a constraint to internal turgor1,3. In Gram-positive bacteria, peptidoglycan is tens of nanometres thick, generally portrayed as a homogeneous structure that provides mechanical strength4-6. Here we applied atomic force microscopy7-12 to interrogate the morphologically distinct Staphylococcus aureus and Bacillus subtilis species, using live cells and purified peptidoglycan. The mature surface of live cells is characterized by a landscape of large (up to 60 nm in diameter), deep (up to 23 nm) pores constituting a disordered gel of peptidoglycan. The inner peptidoglycan surface, consisting of more nascent material, is much denser, with glycan strand spacing typically less than 7 nm. The inner surface architecture is location dependent; the cylinder of B. subtilis has dense circumferential orientation, while in S. aureus and division septa for both species, peptidoglycan is dense but randomly oriented. Revealing the molecular architecture of the cell envelope frames our understanding of its mechanical properties and role as the environmental interface13,14, providing information complementary to traditional structural biology approaches.
Conflict of interest statement
The authors declare no financial or non-financial competing interests.
Figures










Similar articles
-
Cell wall peptidoglycan architecture in Bacillus subtilis.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14603-8. doi: 10.1073/pnas.0804138105. Epub 2008 Sep 10. Proc Natl Acad Sci U S A. 2008. PMID: 18784364 Free PMC article.
-
Atomic Force Microscopy Analysis of Bacterial Cell Wall Peptidoglycan Architecture.Methods Mol Biol. 2016;1440:3-9. doi: 10.1007/978-1-4939-3676-2_1. Methods Mol Biol. 2016. PMID: 27311660
-
Architecture and assembly of the Gram-positive cell wall.Mol Microbiol. 2013 May;88(4):664-72. doi: 10.1111/mmi.12203. Epub 2013 Apr 22. Mol Microbiol. 2013. PMID: 23600697 Free PMC article.
-
The linkage between teichoic acid and peptidoglycan in bacterial cell walls.FEBS Lett. 1978 Apr 1;88(1):1-9. doi: 10.1016/0014-5793(78)80594-8. FEBS Lett. 1978. PMID: 416968 Review. No abstract available.
-
Bacteria's different ways to recycle their own cell wall.Int J Med Microbiol. 2019 Nov;309(7):151326. doi: 10.1016/j.ijmm.2019.06.006. Epub 2019 Jul 4. Int J Med Microbiol. 2019. PMID: 31296364 Review.
Cited by
-
Bacterial Targets of Antibiotics in Methicillin-Resistant Staphylococcus aureus.Antibiotics (Basel). 2021 Apr 7;10(4):398. doi: 10.3390/antibiotics10040398. Antibiotics (Basel). 2021. PMID: 33917043 Free PMC article. Review.
-
Macromolecular Crowding, Phase Separation, and Homeostasis in the Orchestration of Bacterial Cellular Functions.Chem Rev. 2024 Feb 28;124(4):1899-1949. doi: 10.1021/acs.chemrev.3c00622. Epub 2024 Feb 8. Chem Rev. 2024. PMID: 38331392 Free PMC article. Review.
-
Rejuvenating the Activity of Usual Antibiotics on Resistant Gram-Negative Bacteria: Recent Issues and Perspectives.Int J Mol Sci. 2023 Jan 12;24(2):1515. doi: 10.3390/ijms24021515. Int J Mol Sci. 2023. PMID: 36675027 Free PMC article. Review.
-
Bioinformatic prediction of proteins relevant to functions of the bacterial OLE ribonucleoprotein complex.mSphere. 2024 Jun 25;9(6):e0015924. doi: 10.1128/msphere.00159-24. Epub 2024 May 21. mSphere. 2024. PMID: 38771028 Free PMC article.
-
Hydration solids.Nature. 2023 Jul;619(7970):500-505. doi: 10.1038/s41586-023-06144-y. Epub 2023 Jun 7. Nature. 2023. PMID: 37286609 Free PMC article.
References
-
- Vollmer W, Seligman SJ. Architecture of peptidoglycan: more data and more models. Trends Microbiol. 2010;18:59–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

