Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus
- PMID: 32523312
- PMCID: PMC7265139
- DOI: 10.3748/wjg.v26.i20.2570
Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus
Abstract
Background: Circulating microRNAs (miRNAs) are potential biomarkers for many diseases. However, they can originate from non-disease specific sources, such as blood cells, and compromise the investigations for miRNA biomarkers. While small extracellular vesicles (sEVs) have been suggested to provide a purer source of circulating miRNAs for biomarkers discovery, the most suitable blood sample for sEV miRNA biomarker studies has not been defined.
Aim: To compare the miRNA profiles between matched serum and plasma sEV preparations to determine their suitability for biomarker studies.
Methods: Matched serum and plasma samples were obtained from 10 healthy controls and 10 patients with esophageal adenocarcinoma. sEV isolates were prepared from serum and plasma using ExoQuickTM and quantified using NanoSight. RNA was extracted from sEV preparations with the miRNeasy Serum/Plasma kit and profiled using the Taqman Openarray qPCR. The overall miRNA content and the expression of specific miRNAs of reported vesicular and non-vesicular origins were compared between serum and plasma sEV preparations. The diagnostic performance of a previously identified multi-miRNA biomarker panel for esophageal adenocarcinoma was also compared.
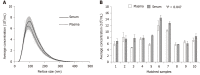
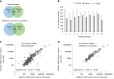
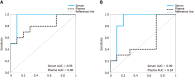
Results: The overall miRNA content was higher in plasma sEV preparations (480 miRNAs) and contained 97.5% of the miRNAs found in the serum sEV preparations (412 miRNAs).The expression of commonly expressed miRNAs was highly correlated (Spearman's R = 0.87, P < 0.0001) between the plasma and serum sEV preparations, but was consistently higher in the plasma sEV preparations. Specific blood-cell miRNAs (hsa-miR-223-3p, hsa-miR-451a, miR-19b-3p, hsa-miR-17-5p, hsa-miR-30b-5p, hsa-miR-106a-5p, hsa-miR-150-5p and hsa-miR-92a-3p) were expressed at 2.7 to 9.6 fold higher levels in the plasma sEV preparations compared to serum sEV preparations (P < 0.05). In plasma sEV preparations, the percentage of protein-associated miRNAs expressed at relatively higher levels (Ct 20-25) was greater than serum sEV preparations (50% vs 31%). While the percentage of vesicle-associated miRNAs expressed at relatively higher levels was greater in the serum sEV preparations than plasma sEV preparations (70% vs 44%). A 5-miRNA biomarker panel produced a higher cross validated accuracy for discriminating patients with esophageal adenocarcinoma from healthy controls using serum sEV preparations compared with plasma sEV preparations (AUROC 0.80 vs 0.54, P < 0.05).
Conclusion: Although plasma sEV preparations contained more miRNAs than serum sEV preparations, they also contained more miRNAs from non-vesicle origins. Serum appears to be more suitable than plasma for sEV miRNAs biomarkers studies.
Keywords: Adenocarcinoma of esophagus; Biomarkers; Blood cells; Circulating microRNA; Exosomes; Extracellular vesicles; MicroRNAs; Plasma; Real-time polymerase chain reaction; Serum.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Figures



Similar articles
-
Identification of non-invasive biomarkers for chronic atrophic gastritis from serum exosomal microRNAs.BMC Cancer. 2019 Feb 8;19(1):129. doi: 10.1186/s12885-019-5328-7. BMC Cancer. 2019. PMID: 30736753 Free PMC article.
-
Circulating Serum Exosomal miRNAs As Potential Biomarkers for Esophageal Adenocarcinoma.J Gastrointest Surg. 2015 Jul;19(7):1208-15. doi: 10.1007/s11605-015-2829-9. Epub 2015 May 6. J Gastrointest Surg. 2015. PMID: 25943911
-
Identification of a 7-microRNA signature in plasma as promising biomarker for nasopharyngeal carcinoma detection.Cancer Med. 2020 Feb;9(3):1230-1241. doi: 10.1002/cam4.2676. Epub 2019 Dec 19. Cancer Med. 2020. PMID: 31856390 Free PMC article.
-
The role of small extracellular vesicle-miRNAs in endometriosis.Hum Reprod. 2023 Dec 4;38(12):2296-2311. doi: 10.1093/humrep/dead216. Hum Reprod. 2023. PMID: 37877421 Free PMC article. Review.
-
Identification of differentially expressed circulating serum microRNA for the diagnosis and prognosis of Indian non-small cell lung cancer patients.Curr Probl Cancer. 2020 Aug;44(4):100540. doi: 10.1016/j.currproblcancer.2020.100540. Epub 2020 Jan 23. Curr Probl Cancer. 2020. PMID: 32007320 Review.
Cited by
-
MicroRNA Profiling in Oesophageal Adenocarcinoma Cell Lines and Patient Serum Samples Reveals a Role for miR-451a in Radiation Resistance.Int J Mol Sci. 2020 Nov 24;21(23):8898. doi: 10.3390/ijms21238898. Int J Mol Sci. 2020. PMID: 33255413 Free PMC article.
-
Development of a Sampling and Storage Protocol of Extracellular Vesicles (EVs)-Establishment of the First EV Biobank for Polytraumatized Patients.Int J Mol Sci. 2024 May 22;25(11):5645. doi: 10.3390/ijms25115645. Int J Mol Sci. 2024. PMID: 38891833 Free PMC article.
-
Clinical Value and Molecular Function of Circulating MicroRNAs in Endometrial Cancer Regulation: A Systematic Review.Cells. 2022 Jun 3;11(11):1836. doi: 10.3390/cells11111836. Cells. 2022. PMID: 35681531 Free PMC article.
-
Cross validated serum small extracellular vesicle microRNAs for the detection of oropharyngeal squamous cell carcinoma.J Transl Med. 2020 Jul 10;18(1):280. doi: 10.1186/s12967-020-02446-1. J Transl Med. 2020. PMID: 32650803 Free PMC article.
-
Circulating miR-122-5p, miR-151a-3p, miR-126-5p and miR-21-5p as potential predictive biomarkers for Metabolic Dysfunction-Associated Steatotic Liver Disease assessment.J Physiol Biochem. 2024 Aug 14. doi: 10.1007/s13105-024-01037-8. Online ahead of print. J Physiol Biochem. 2024. PMID: 39138826
References
-
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

