Successful muscle regeneration by a homologous microperforated scaffold seeded with autologous mesenchymal stromal cells in a porcine esophageal substitution model
- PMID: 32523626
- PMCID: PMC7257852
- DOI: 10.1177/1756284820923220
Successful muscle regeneration by a homologous microperforated scaffold seeded with autologous mesenchymal stromal cells in a porcine esophageal substitution model
Abstract
Background: Since the esophagus has no redundancy, congenital and acquired esophageal diseases often require esophageal substitution, with complicated surgery and intestinal or gastric transposition. Peri-and-post-operative complications are frequent, with major problems related to the food transit and reflux. During the last years tissue engineering products became an interesting therapeutic alternative for esophageal replacement, since they could mimic the organ structure and potentially help to restore the native functions and physiology. The use of acellular matrices pre-seeded with cells showed promising results for esophageal replacement approaches, but cell homing and adhesion to the scaffold remain an important issue and were investigated.
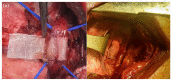
Methods: A porcine esophageal substitute constituted of a decellularized scaffold seeded with autologous bone marrow-derived mesenchymal stromal cells (BM-MSCs) was developed. In order to improve cell seeding and distribution throughout the scaffolds, they were micro-perforated by Quantum Molecular Resonance (QMR) technology (Telea Electronic Engineering).
Results: The treatment created a microporous network and cells were able to colonize both outer and inner layers of the scaffolds. Non seeded (NSS) and BM-MSCs seeded scaffolds (SS) were implanted on the thoracic esophagus of 4 and 8 pigs respectively, substituting only the muscle layer in a mucosal sparing technique. After 3 months from surgery, we observed an esophageal substenosis in 2/4 NSS pigs and in 6/8 SS pigs and a non-practicable stricture in 1/4 NSS pigs and 2/8 SS pigs. All the animals exhibited a normal weight increase, except one case in the SS group. Actin and desmin staining of the post-implant scaffolds evidenced the regeneration of a muscular layer from one anastomosis to another in the SS group but not in the NSS one.
Conclusions: A muscle esophageal substitute starting from a porcine scaffold was developed and it was fully repopulated by BM-MSCs after seeding. The substitute was able to recapitulate in shape and function the original esophageal muscle layer.
Keywords: 3D cell culture; Quantum Molecular Resonance; esophagus; mesenchymal stromal cells; scaffold; tissue engineering.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: GC is a consultant for Olympus, Cook Medical, and Boston Scientific. IB is a research grant holder from Apollo Endosurgery and a consultant for Apollo Endosurgery, Cook Medical, and Boston Scientific.
Figures




References
-
- Ludman L, Spitz L. Quality of life after gastric transposition for oesophageal atresia. J Pediatr Surg 2003; 38: 53–57. - PubMed
-
- Ure BM, Slany E, Eypasch EP, et al. Long- term functional results and quality of life after colon interposition for long-gap oesophageal atresia. Eur J Pediatr Surg 1995; 5: 206–210. - PubMed
-
- Awad K, Jaffray B. Oesophageal replacement with stomach: a personal series and review of published experience. J Paediatr Child Health 2017; 53: 1159–1166. - PubMed
-
- Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg 2000; 119: 1126–1132. - PubMed
-
- Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005; 201: 253–262. - PubMed
LinkOut - more resources
Full Text Sources

