Screening of a Library of Recombinant Schistosoma mansoni Proteins With Sera From Murine and Human Controlled Infections Identifies Early Serological Markers
- PMID: 32524140
- PMCID: PMC9016452
- DOI: 10.1093/infdis/jiaa329
Screening of a Library of Recombinant Schistosoma mansoni Proteins With Sera From Murine and Human Controlled Infections Identifies Early Serological Markers
Abstract
Background: Schistosomiasis is a major global health problem caused by blood-dwelling parasitic worms, which is currently tackled primarily by mass administration of the drug praziquantel. Appropriate drug treatment strategies are informed by diagnostics that establish the prevalence and intensity of infection, which, in regions of low transmission, should be highly sensitive.
Methods: To identify sensitive new serological markers of Schistosoma mansoni infections, we have compiled a recombinant protein library of parasite cell-surface and secreted proteins expressed in mammalian cells.
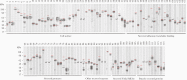
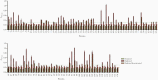
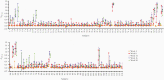
Results: Together with a time series of sera samples from volunteers experimentally infected with a defined number of male parasites, we probed this protein library to identify several markers that can detect primary infections with as low as 10 parasites and as early as 5 weeks postinfection.
Conclusions: These new markers could be further explored as valuable tools to detect ongoing and previous S mansoni infections, including in endemic regions where transmission is low.
Keywords: Schistosoma mansoni; antibodies; schistosomiasis; serology.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- World Health Organization. Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2016. Wkly Epidemiol Rec 2017; 92:749–60. - PubMed
-
- Cousin CE, Stirewalt MA, Dorsey CH. Schistosoma mansoni: ultrastructure of early transformation of skin- and shear-pressure-derived schistosomules. Exp Parasitol 1981; 51:341–65. - PubMed
-
- Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of egg production and egg excretion by Schistosoma mansoni and S. japonicum in mice infected with a single pair of worms. Am J Trop Med Hyg 1994; 50:281–95. - PubMed
-
- Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol 2009; 31:163–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

