A Review of Two Regulatory Approved Anti-CD19 CAR T-Cell Therapies in Diffuse Large B-Cell Lymphoma: Why Are Indirect Treatment Comparisons Not Feasible?
- PMID: 32524498
- PMCID: PMC7467403
- DOI: 10.1007/s12325-020-01397-9
A Review of Two Regulatory Approved Anti-CD19 CAR T-Cell Therapies in Diffuse Large B-Cell Lymphoma: Why Are Indirect Treatment Comparisons Not Feasible?
Abstract
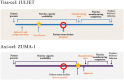
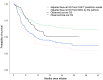
Anti-CD19 chimeric antigen receptor (CAR) T-cell therapies can be effective for diffuse large B-cell lymphoma (DLBCL), a cancer with limited treatment options and poor outcomes, particularly for patients with relapsed or refractory (r/r) disease. Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) are CAR T-cell therapies approved by regulatory bodies for certain patients with r/r DLBCL on the basis of demonstrated treatment effects in their pivotal single-arm trials, ZUMA-1 and JULIET, respectively. In the absence of head-to-head trials, the question of whether a valid indirect treatment comparison (ITC) between axi-cel and tisa-cel could be performed using existing evidence is of interest to patients, physicians, payers, and other stakeholders. This article addresses that question by summarizing the current evidence from clinical trials and real-world studies and discussing the challenges and limitations of potential analytical approaches associated with an ITC. Two ITC approaches attempting to adjust for cross-trial heterogeneity between ZUMA-1 and JULIET, matching-adjusted indirect comparison and regression-prediction model analysis, were evaluated. After evaluating the current clinical trial data and real-world evidence, and present and prior ITC analyses of axi-cel and tisa-cel, the authors conclude that a valid comparative analysis is not currently feasible. The substantial differences (e.g., timing of leukapheresis and enrollment, use of bridging chemotherapy [90% in JULIET vs. 0% in ZUMA-1], lymphodepleting regimens) between the two trials' designs and patient populations preclude a robust and reliable ITC. No other approaches are able to account for such differences. The current real-world data are still too immature to be used for ITCs. Thus, drawing conclusions from such ITCs should be avoided to prevent misinforming treatment choices or limiting patient access to effective treatment options. Additional data from ongoing or future real-world studies with appropriate statistical analyses are needed to provide insights into the comparative effectiveness and safety of these two treatments.
Keywords: Anti-CD19 chimeric antigen receptor T-cell therapies; Axicabtagene ciloleucel; Diffuse large B-cell lymphoma; Indirect treatment comparison; Tisagenlecleucel.
Figures
References
-
- World Health Organization. Diffuse large B-cell lymphoma. https://www.who.int/selection_medicines/committees/expert/20/application.... Accessed 4 Apr 2020
-
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA-Cancer J Clin. 2016;66(6):443–459. - PubMed
-
- National Cancer Institute. Cancer stat facts: NHL- diffuse large B-call lymphoma (DLBCL). Available at: https://seer.cancer.gov/statfacts/html/dlbcl.html. Accessed 4 Apr 2020.
-
- Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–v125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

