Normal and Abnormal Postoperative Imaging Findings after Gastric Oncologic and Bariatric Surgery
- PMID: 32524781
- PMCID: PMC7289697
- DOI: 10.3348/kjr.2019.0822
Normal and Abnormal Postoperative Imaging Findings after Gastric Oncologic and Bariatric Surgery
Abstract
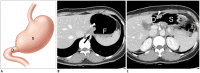
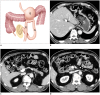
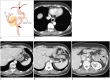
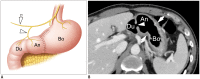
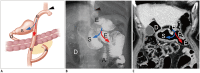
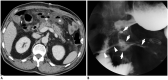
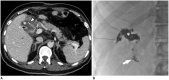
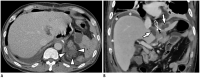
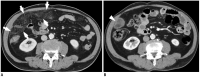
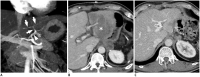
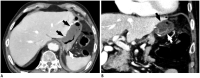
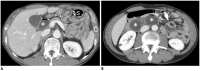
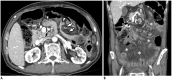
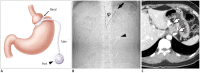
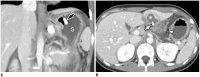
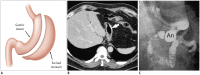
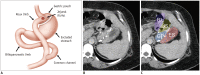
Surgical resection remains the primary choice of treatment and the only potentially curative option for gastric carcinoma, and is increasingly performed laparoscopically. Gastric resection represents a challenging procedure, with a significant morbidity and non-negligible postoperative mortality. The interpretation of imaging after gastric surgery can be challenging due to significant modifications of the normal anatomy. After the surgery, the familiarity with expected imaging appearances is crucial for diagnosis and appropriate management of potentially life-threatening complications in patients who underwent gastric surgery. We review various surgical techniques used in gastric surgery and describe fluoroscopic and cross-sectional imaging appearances of normal postoperative anatomic changes as well as early and late complications after gastric surgery.
Keywords: CT; Postoperative complications; Stomach; Stomach cancer; Surgical techniques.
Copyright © 2020 The Korean Society of Radiology.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures


References
-
- Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Lee DH, Kim SH, Joo I, Hur BY, Han JK. Comparison between 18F-FDG PET/MRI and MDCT for the assessment of preoperative staging and resectability of gastric cancer. Eur J Radiol. 2016;85:1085–1091. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

