Biophysical basis for Kv1.3 regulation of membrane potential changes induced by P2X4-mediated calcium entry in microglia
- PMID: 32525239
- PMCID: PMC7540709
- DOI: 10.1002/glia.23847
Biophysical basis for Kv1.3 regulation of membrane potential changes induced by P2X4-mediated calcium entry in microglia
Abstract
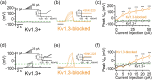
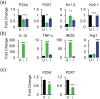
Microglia-mediated inflammation exerts adverse effects in ischemic stroke and in neurodegenerative disorders such as Alzheimer's disease (AD). Expression of the voltage-gated potassium channel Kv1.3 is required for microglia activation. Both genetic deletion and pharmacological inhibition of Kv1.3 are effective in reducing microglia activation and the associated inflammatory responses, as well as in improving neurological outcomes in animal models of AD and ischemic stroke. Here we sought to elucidate the molecular mechanisms underlying the therapeutic effects of Kv1.3 inhibition, which remain incompletely understood. Using a combination of whole-cell voltage-clamp electrophysiology and quantitative PCR (qPCR), we first characterized a stimulus-dependent differential expression pattern for Kv1.3 and P2X4, a major ATP-gated cationic channel, both in vitro and in vivo. We then demonstrated by whole-cell current-clamp experiments that Kv1.3 channels contribute not only to setting the resting membrane potential but also play an important role in counteracting excessive membrane potential changes evoked by depolarizing current injections. Similarly, the presence of Kv1.3 channels renders microglia more resistant to depolarization produced by ATP-mediated P2X4 receptor activation. Inhibiting Kv1.3 channels with ShK-223 completely nullified the ability of Kv1.3 to normalize membrane potential changes, resulting in excessive depolarization and reduced calcium transients through P2X4 receptors. Our report thus links Kv1.3 function to P2X4 receptor-mediated signaling as one of the underlying mechanisms by which Kv1.3 blockade reduces microglia-mediated inflammation. While we could confirm previously reported differences between males and females in microglial P2X4 expression, microglial Kv1.3 expression exhibited no gender differences in vitro or in vivo. MAIN POINTS: The voltage-gated K+ channel Kv1.3 regulates microglial membrane potential. Inhibition of Kv1.3 depolarizes microglia and reduces calcium entry mediated by P2X4 receptors by dissipating the electrochemical driving force for calcium.
Keywords: Kir2.1; Kv1.3; P2X4; P2X7; PAP-1; ShK-223; intracellular Ca2+; membrane potential; microglia; potassium channels; purinergic receptor.
© 2020 The Authors. Glia published by Wiley Periodicals LLC.
Figures

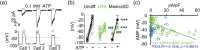




References
-
- Beeton, C. , Wulff, H. , Standifer, N. E. , Azam, P. , Mullen, K. M. , Pennington, M. W. , … Chandy, K. G. (2006). Kv1.3 channels are a therapeutic target for T cell‐mediated autoimmune diseases. Proceedings of the National Academy of Sciences of the United States of America, 103(46), 17414–17419. - PMC - PubMed
-
- Boucsein, C. , Zacharias, R. , Farber, K. , Pavlovic, S. , Hanisch, U. K. , & Kettenmann, H. (2003). Purinergic receptors on microglial cells: Functional expression in acute brain slices and modulation of microglial activation in vitro. The European Journal of Neuroscience, 17(11), 2267–2276. 10.1046/j.1460-9568.2003.02663.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

