Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema
- PMID: 32526076
- PMCID: PMC7116441
- DOI: 10.1002/stem.3241
Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema
Abstract
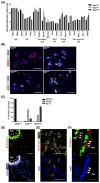
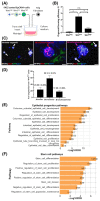
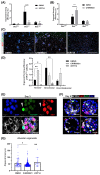
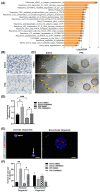
Wnt/β-catenin signaling regulates progenitor cell fate decisions during lung development and in various adult tissues. Ectopic activation of Wnt/β-catenin signaling promotes tissue repair in emphysema, a devastating lung disease with progressive loss of parenchymal lung tissue. The identity of Wnt/β-catenin responsive progenitor cells and the potential impact of Wnt/β-catenin signaling on adult distal lung epithelial progenitor cell function in emphysema are poorly understood. Here, we used TCF/Lef:H2B/GFP reporter mice to investigate the role of Wnt/β-catenin signaling in lung organoid formation. We identified an organoid-forming adult distal lung epithelial progenitor cell population characterized by a low Wnt/β-catenin activity, which was enriched in club and alveolar epithelial type (AT)II cells. Endogenous Wnt/β-catenin activity was required for the initiation of multiple subtypes of distal lung organoids derived from the Wntlow epithelial progenitors. Further ectopic Wnt/β-catenin activation specifically led to an increase in alveolar organoid number; however, the subsequent proliferation of alveolar epithelial cells in the organoids did not require constitutive Wnt/β-catenin signaling. Distal lung epithelial progenitor cells derived from the mouse model of elastase-induced emphysema exhibited reduced organoid forming capacity. This was rescued by Wnt/β-catenin signal activation, which largely increased the number of alveolar organoids. Together, our study reveals a novel mechanism of lung epithelial progenitor cell activation in homeostasis and emphysema.
Keywords: Wnt/β-catenin; chronic lung disease; emphysema; lung epithelial progenitor; organoid; regeneration.
©2020 The Authors. Stem Cells published by Wiley Periodicals LLC on behalf of AlphaMed Press 2020.
Conflict of interest statement
R.G. declared research grants from Boehringer Ingelheim, Aquilo, Chiesi, and Novartis. The other authors declared no potential conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

