Clostridioides difficile Senses and Hijacks Host Heme for Incorporation into an Oxidative Stress Defense System
- PMID: 32526159
- PMCID: PMC7486240
- DOI: 10.1016/j.chom.2020.05.015
Clostridioides difficile Senses and Hijacks Host Heme for Incorporation into an Oxidative Stress Defense System
Abstract
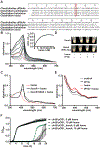
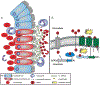
Clostridioides difficile infection of the colon leads to severe inflammation and damage to the gastrointestinal epithelium due to the production of potent toxins. This inflammatory tissue damage causes the liberation of high concentrations of host heme at infection sites. Here, we identify the C. difficile heme-sensing membrane protein system (HsmRA) and show that this operon induces a protective response that repurposes heme to counteract antimicrobial oxidative stress responses. HsmR senses vertebrate heme, leading to increased expression of the hsmRA operon and subsequent deployment of HsmA to capture heme and reduce redox damage caused by inflammatory mediators of protection and antibiotic therapy. Strains with inactivated hsmR or hsmA have increased sensitivity to redox-active compounds and reduced colonization persistence in a murine model of relapse C. difficile infection. These results define a mechanism exploited by C. difficile to repurpose toxic heme within the inflamed gut as a shield against antimicrobial compounds.
Keywords: Clostridioides difficile; antibiotic sensitivity; heme utilization; host-pathogen interactions; nutritional immunity; relapse infection.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests Authors declare no competing interests.
Figures




Comment in
-
Clostridioides difficile: Sometimes It Pays To Be Difficult.Cell Host Microbe. 2020 Sep 9;28(3):358-359. doi: 10.1016/j.chom.2020.08.010. Cell Host Microbe. 2020. PMID: 32910918
References
-
- Benjamini Y, and Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 57, 289–300.
-
- Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, et al. (1997). The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

