Efficacy, duration of protection, birth outcomes, and infant growth associated with influenza vaccination in pregnancy: a pooled analysis of three randomised controlled trials
- PMID: 32526188
- PMCID: PMC7284303
- DOI: 10.1016/S2213-2600(19)30479-5
Efficacy, duration of protection, birth outcomes, and infant growth associated with influenza vaccination in pregnancy: a pooled analysis of three randomised controlled trials
Abstract
Background: Maternal influenza immunisation can reduce morbidity and mortality associated with influenza infection in pregnant women and young infants. We aimed to determine the vaccine efficacy of maternal influenza immunisation against maternal and infant PCR-confirmed influenza, duration of protection, and the effect of gestational age at vaccination on vaccine efficacy, birth outcomes, and infant growth up to 6 months of age.
Methods: We did a pooled analysis of three randomised controlled trials done in Nepal (2011-2014), Mali (2011-2014), and South Africa (2011-2013). Pregnant women, gestational age 17-34 weeks in Nepal, 28 weeks or more in Mali, and 20-36 weeks in South Africa, were enrolled. Women were randomly assigned 1:1 to a study group, in which they received trivalent inactivated influenza vaccine (IIV) in all three trials, or a control group, in which they received saline placebo in Nepal and South Africa or quadrivalent meningococcal conjugate vaccine in Mali. Enrolment at all sites was complete by April 24, 2013. Infants and women were assessed for respiratory illness, and samples from those that met the case definition were tested for influenza by PCR testing. Growth measurements, including length and weight, were obtained at birth at all sites, at 24 weeks in South Africa, and at 6 months in Nepal and Mali. The three trials are registered with ClinicalTrials.gov, numbers NCT01430689, NCT01034254, and NCT02465190.
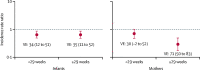
Findings: 10 002 women and 9800 liveborn infants were included. Pooled efficacy of maternal vaccination to prevent infant PCR-confirmed influenza up to 6 months of age was 35% (95% CI 19 to 47). The pooled estimate was 56% (28 to 73) within the first 2 months of life, 39% (11 to 58) between 2 and 4 months, and 19% (-9 to 40) between 4 and 6 months. In women, from enrolment during pregnancy to the end of follow-up at 6 months postpartum, the vaccine was 50% (95% CI 32-63) efficacious against PCR-confirmed influenza. Efficacy was 42% (12 to 61) during pregnancy and 60% (36 to 75) postpartum. In women vaccinated before 29 weeks gestational age, the estimated efficacy was 30% (-2 to 52), and in women vaccinated at or after 29 weeks, efficacy was 71% (50 to 83). Efficacy was similar in infants born to mothers vaccinated before or after 29 weeks gestation (34% [95% CI 12 to 51] vs 35% [11 to 52]). There was no overall association between maternal vaccination and low birthweight, stillbirth, preterm birth, and small for gestational age. At 6 months of age, the intervention and control groups were similar in terms of underweight (weight-for-age), stunted (length-for-age), and wasted (weight-for-length). Median centile change from birth to 6 months of age was similar between the intervention and the control groups for both weight and length.
Interpretation: The assessment of efficacy for women vaccinated before 29 weeks gestational age might have been underpowered, because the point estimate suggests that there might be efficacy despite wide CIs. Estimates of efficacy against PCR-confirmed influenza and safety in terms of adverse birth outcomes should be incorporated into any further consideration of maternal influenza immunisation recommendations.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Influenza immunisation in pregnancy is efficacious and safe, but questions remain.Lancet Respir Med. 2020 Jun;8(6):533-534. doi: 10.1016/S2213-2600(20)30034-5. Lancet Respir Med. 2020. PMID: 32526185 No abstract available.
References
-
- Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207(suppl):S3–S8. - PubMed
-
- Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010–2016. Pediatrics. 2018;141 - PubMed
-
- Zaman K, Roy E, Arifeen SE. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–1564. - PubMed
-
- Omer SB, Richards JL, Madhi SA. Three randomized trials of maternal influenza immunization in Mali, Nepal, and South Africa: methods and expectations. Vaccine. 2015;33:3801–3812. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

