Targeting CD33 in Chemoresistant AML Patient-Derived Xenografts by CAR-CIK Cells Modified with an Improved SB Transposon System
- PMID: 32526203
- PMCID: PMC7474266
- DOI: 10.1016/j.ymthe.2020.05.021
Targeting CD33 in Chemoresistant AML Patient-Derived Xenografts by CAR-CIK Cells Modified with an Improved SB Transposon System
Abstract
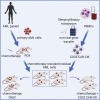
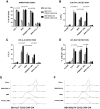
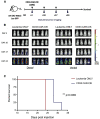
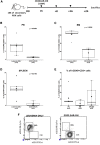
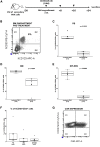
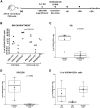
The successful implementation of chimeric antigen receptor (CAR)-T cell therapy in the clinical context of B cell malignancies has paved the way for further development in the more critical setting of acute myeloid leukemia (AML). Among the potentially targetable AML antigens, CD33 is insofar one of the main validated molecules. Here, we describe the feasibility of engineering cytokine-induced killer (CIK) cells with a CD33.CAR by using the latest optimized version of the non-viral Sleeping Beauty (SB) transposon system "SB100X-pT4." This offers the advantage of improving CAR expression on CIK cells, while reducing the amount of DNA transposase as compared to the previously employed "SB11-pT" version. SB-modified CD33.CAR-CIK cells exhibited significant antileukemic activity in vitro and in vivo in patient-derived AML xenograft models, reducing AML development when administered as an "early treatment" and delaying AML progression in mice with established disease. Notably, by exploiting an already optimized xenograft chemotherapy model that mimics human induction therapy in mice, we demonstrated for the first time that CD33.CAR-CIK cells are also effective toward chemotherapy resistant/residual AML cells, further supporting its future clinical development and implementation within the current standard regimens.
Keywords: AML; CAR; CD33; Sleeping Beauty transposon; cytokine-induced killer cells; immunotherapy; non-viral gene transfer.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

