High rates of 30-day mortality in patients with cirrhosis and COVID-19
- PMID: 32526252
- PMCID: PMC7280108
- DOI: 10.1016/j.jhep.2020.06.001
High rates of 30-day mortality in patients with cirrhosis and COVID-19
Abstract
Background & aims: Coronavirus disease 2019 (COVID-19) poses a major health threat to healthy individuals and those with comorbidities, but its impact on patients with cirrhosis is currently unknown. Herein, we aimed to evaluate the impact of COVID-19 on the clinical outcome of patients with cirrhosis.
Methods: In this multicentre retrospective study, patients with cirrhosis and a confirmed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection were enrolled between 1st and 31th March 2020. Clinical and biochemical data at diagnosis of COVID-19 and at the last outpatient visit were obtained through review of medical records.
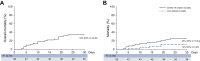
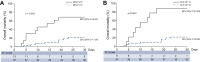
Results: Fifty patients with cirrhosis and confirmed SARS-CoV-2 infection were enrolled (age 67 years, 70% men, 38% virus-related, 52% previously compensated cirrhosis). At diagnosis, 64% of patients presented fever, 42% shortness of breath/polypnea, 22% encephalopathy, 96% needed hospitalization or a prolonged stay if already in hospital. Respiratory support was necessary in 71%, 52% received antivirals, 80% heparin. Serum albumin significantly decreased, while bilirubin, creatinine and prothrombin time significantly increased at COVID-19 diagnosis compared to last available data. The proportion of patients with a model for end-stage liver disease (MELD) score ≥15 increased from 13% to 26% (p = 0.037), acute-on-chronic liver failure and de novo acute liver injury occurred in 14 (28%) and 10 patients, respectively. Seventeen patients died after a median of 10 (4-13) days from COVID-19 diagnosis, with a 30-day-mortality rate of 34%. The severity of lung and liver (according to CLIF-C, CLIF-OF and MELD scores) diseases independently predicted mortality. In patients with cirrhosis, mortality was significantly higher in those with COVID-19 than in those hospitalized for bacterial infections.
Conclusion: COVID-19 is associated with liver function deterioration and elevated mortality in patients with cirrhosis.
Lay summary: Coronavirus disease 2019 (COVID-19) poses a major health threat to healthy individuals and those with comorbidities. Herein, we assessed its impact on patients with cirrhosis. Infection with COVID-19 was associated with liver function deterioration and elevated mortality in patients with cirrhosis.
Keywords: HBV; HCV; Hepatitis; Hepatocellular carcinoma; Liver transplantation; SARS-CoV-2.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest Massimo Iavarone: Speaking/Teaching, consultant and advisory board for Bayer, Gilead Sciences, BMS, Janssen, Ipsen, MSD, BTG-Boston Scientific, AbbVie, Guerbet, EISAI; Roberta D'Ambrosio: teaching and speaking for AbbVie, Gilead, MSD; Advisory Board for AbbVie, MSD, Research Grant from Gilead; Alessandro Soria: Speaking/Teaching, consultant and advisory board for AbbVie, MSD, Gilead; Mauro Viganò: speaking and teaching for Fujirebio, Intercept, Gilead; Alessio Aghemo: Advisory Board/Speaker Bureau for: Gilead, AbbVie, Intercept, MSD, Mylan and Alfasigma, Research grants from Gilead and Abbvie; Stefano Fagiuoli: Advisory Board/Speaker Bureau for Gilead, AbbVie, Novartis, MSD, Bayer, Intercept, Kedrion; Pietro Invernizzi: Advisory Board/Speaker Bureau for Gilead, Intercept, Bruschettini, AbbVie, MSD; Pietro Lampertico: Advisory Board/Speaker Bureau for BMS, Roche, Gilead, GSK, AbbVie, MSD, Arrowhead, Alnylam, Janssen, Spring Bank, MYR, Eiger. The other authors declare no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Comment in
-
Letter regarding "High rates of 30-day mortality in patients with cirrhosis and COVID-19".J Hepatol. 2020 Dec;73(6):1569-1570. doi: 10.1016/j.jhep.2020.06.023. Epub 2020 Jun 20. J Hepatol. 2020. PMID: 32574577 Free PMC article. No abstract available.
-
The impact of COVID-19 on the clinical outcome of patients with cirrhosis deserves more attention and research.J Hepatol. 2020 Dec;73(6):1568-1569. doi: 10.1016/j.jhep.2020.06.024. Epub 2020 Jun 20. J Hepatol. 2020. PMID: 32574579 Free PMC article. No abstract available.
References
-
- World Health Organization Novel coronavirus — China. January 12, 2020. http://www.who.int/csr/don/12%5fjanuary-2020-novel-coronavirus-china/en/
-
- Istituto Superiore di Sanità “COVID-19 epidemic. 2 April 2020 national update” Appendix to the bulletin with a regional breakdown. https://www.epicentro.iss.it/coronavirus/bollettino/Bolletino-sorveglian...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

