A large number of COVID-19 interventional clinical trials were registered soon after the pandemic onset: a descriptive analysis
- PMID: 32526460
- PMCID: PMC7278640
- DOI: 10.1016/j.jclinepi.2020.06.005
A large number of COVID-19 interventional clinical trials were registered soon after the pandemic onset: a descriptive analysis
Abstract
Background and objective: There is a pressing need for evidence-based interventions to address the devastating clinical and public health effects of the coronavirus disease 2019 (COVID-19) pandemic. The number of registered trials related to COVID-19 is increasing by the day. The objective of this study was to describe the characteristics of the currently registered interventional clinical trials related to COVID-19.
Methods: We searched the World Health Organization's International Clinical Trials Registry Platform on May 15th, 2020. We included any entry that is related to COVID-19. We abstracted and then descriptively analyzed the following characteristics of the registered trials: study design, status, phase, primary endpoints, experimental interventions, and geographic location among other qualifiers.
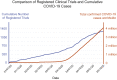
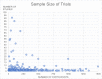
Results: We identified 1,308 eligible registered trials. Most trials were registered with ClinicalTrials.gov (n = 703; 53.7%) and the Chinese Clinical Trial Registry (n = 291; 22.2%). The number of participants to be enrolled across these trials was 734,657, with a median of 110 participants per trial. The most commonly studied intervention category was pharmacologic (n = 763; 58.3%), with antiparasitic medications being the most common subcategory. Although over half of the trials were already recruiting, we identified published peer-reviewed results for only 8 of those trials.
Conclusion: There is a relatively large number of registered trials but with very few results published so far. Although our findings suggest an appropriate initial response by the research community, the real challenge will be to get these trials completed, published, and translated into practice and policy.
Keywords: COVID-19; Clinical trials; Novel coronavirus disease; Trial characteristics; Trial registration.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

References
-
- Millán-Oñate J., Rodriguez-Morales A., Camacho-Moreno G., Mendoza-Ramírez H., Rodríguez-Sabogal I., Álvarez-Moreno C. A new emerging zoonotic virus of concern: the 2019 novel Coronavirus (COVID-19) Infection. 2020;24:187–192.
-
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. bioRxiv; Cold Spring Harbor; NY: 2020. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. 2020.02.07.937862.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

