Poor quality Vβ recombination signal sequences stochastically enforce TCRβ allelic exclusion
- PMID: 32526772
- PMCID: PMC7478721
- DOI: 10.1084/jem.20200412
Poor quality Vβ recombination signal sequences stochastically enforce TCRβ allelic exclusion
Abstract
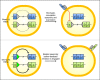
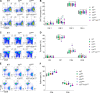
The monoallelic expression of antigen receptor (AgR) genes, called allelic exclusion, is fundamental for highly specific immune responses to pathogens. This cardinal feature of adaptive immunity is achieved by the assembly of a functional AgR gene on one allele, with subsequent feedback inhibition of V(D)J recombination on the other allele. A range of epigenetic mechanisms have been implicated in sequential recombination of AgR alleles; however, we now demonstrate that a genetic mechanism controls this process for Tcrb. Replacement of V(D)J recombinase targets at two different mouse Vβ gene segments with a higher quality target elevates Vβ rearrangement frequency before feedback inhibition, dramatically increasing the frequency of T cells with TCRβ chains derived from both Tcrb alleles. Thus, TCRβ allelic exclusion is enforced genetically by the low quality of Vβ recombinase targets that stochastically restrict the production of two functional rearrangements before feedback inhibition silences one allele.
© 2020 Wu et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures









Comment in
-
RSSs set the odds for exclusion.J Exp Med. 2020 Sep 7;217(9):e20200831. doi: 10.1084/jem.20200831. J Exp Med. 2020. PMID: 32793983 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

