Three-dimensional culture and clinical drug responses of a highly metastatic human ovarian cancer HO-8910PM cells in nanofibrous microenvironments of three hydrogel biomaterials
- PMID: 32527266
- PMCID: PMC7291456
- DOI: 10.1186/s12951-020-00646-x
Three-dimensional culture and clinical drug responses of a highly metastatic human ovarian cancer HO-8910PM cells in nanofibrous microenvironments of three hydrogel biomaterials
Abstract
Background: Ovarian cancer is a highly aggressive malignant disease in gynecologic cancer. It is an urgent task to develop three-dimensional (3D) cell models in vitro and dissect the cell progression-related drug resistance mechanisms in vivo. In the present study, RADA16-I peptide has the reticulated nanofiber scaffold networks in hydrogel, which is utilized to develop robust 3D cell culture of a high metastatic human ovarian cancer HO-8910PM cell line accompanied with the counterparts of Matrigel and collagen I.
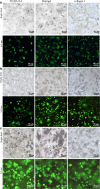
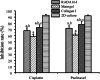
Results: Consequently, HO-8910PM cells were successfully cultivated in three types of hydrogel biomaterials, such as RADA16-I hydrogel, Matrigel, and collagen I, according to 3D cell culture protocols. Designer RADA16-I peptide had well-defined nanofiber networks architecture in hydrogel, which provided nanofiber cell microenvironments analogous to Matrigel and collagen I. 3D-cultured HO-8910PM cells in RADA16-I hydrogel, Matrigel, and collagen I showed viable cell proliferation, proper cell growth, and diverse cell shapes in morphology at the desired time points. For a long 3D cell culture period, HO-8910PM cells showed distinct cell aggregate growth patterns in RADA16-I hydrogel, Matrigel, and collagen I, such as cell aggregates, cell colonies, cell clusters, cell strips, and multicellular tumor spheroids (MCTS). The cell distribution and alignment were described vigorously. Moreover, the molecular expression of integrin β1, E-cadherin and N-cadherin were quantitatively analyzed in 3D-cultured MCTS of HO-8910PM cells by immunohistochemistry and western blotting assays. The chemosensitivity assay for clinical drug responses in 3D context indicated that HO-8910PM cells in three types of hydrogels showed significantly higher chemoresistance to cisplatin and paclitaxel compared to 2D flat cell culture, including IC50 values and inhibition rates.
Conclusion: Based on these results, RADA16-I hydrogel is a highly competent, high-profile, and proactive nanofiber scaffold to maintain viable cell proliferation and high cell vitality in 3D cell models, which may be particularly utilized to develop useful clinical drug screening platform in vitro.
Keywords: 3D cell culture; Cell growth pattern; Chemosensitivity; HO-8910PM cells; Hydrogel; Nanofiber.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Tanaka M. Design of novel 2D and 3D biointerfaces using self-organization to control cell behavior. Biochim Biophys Acta. 2011;1810:251–258. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

