The functional role of the αM4 transmembrane helix in the muscle nicotinic acetylcholine receptor probed through mutagenesis and coevolutionary analyses
- PMID: 32527728
- PMCID: PMC7415963
- DOI: 10.1074/jbc.RA120.013751
The functional role of the αM4 transmembrane helix in the muscle nicotinic acetylcholine receptor probed through mutagenesis and coevolutionary analyses
Abstract
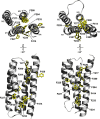
The activity of the muscle-type Torpedo nicotinic acetylcholine receptor (nAChR) is highly sensitive to lipids, but the underlying mechanisms remain poorly understood. The nAChR transmembrane α-helix, M4, is positioned at the perimeter of each subunit in direct contact with lipids and likely plays a central role in lipid sensing. To gain insight into the mechanisms underlying nAChR lipid sensing, we used homology modeling, coevolutionary analyses, site-directed mutagenesis, and electrophysiology to examine the role of the α-subunit M4 (αM4) in the function of the adult muscle nAChR. Ala substitutions for most αM4 residues, including those in clusters of polar residues at both the N and C termini, and deletion of up to 11 C-terminal residues had little impact on the agonist-induced response. Even Ala substitutions for coevolved pairs of residues at the interface between αM4 and the adjacent helices, αM1 and αM3, had little effect, although some impaired nAChR expression. On the other hand, Ala substitutions for Thr422 and Arg429 caused relatively large losses of function, suggesting functional roles for these specific residues. Ala substitutions for aromatic residues at the αM4-αM1/αM3 interface generally led to gains of function, as previously reported for the prokaryotic homolog, the Erwinia chrysanthemi ligand-gated ion channel (ELIC). The functional effects of individual Ala substitutions in αM4 were found to be additive, although not in a completely independent manner. Our results provide insight into the structural features of αM4 that are important. They also suggest how lipid-dependent changes in αM4 structure ultimately modify nAChR function.
Keywords: Cys-loop receptor; ELIC; GLIC; M4; M4 transmembrane helix; channel gating; coevolutionary analysis; lipid sensing; lipid–protein interactions; membrane protein; mutagenesis; nicotinic acetylcholine receptor (nAChR); pentameric ligand-gated ion channel (pLGIC); protein evolution; transmembrane domain.
© 2020 Thompson et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

