miR-107 inhibition upregulates CAB39 and activates AMPK-Nrf2 signaling to protect osteoblasts from dexamethasone-induced oxidative injury and cytotoxicity
- PMID: 32527986
- PMCID: PMC7343481
- DOI: 10.18632/aging.103341
miR-107 inhibition upregulates CAB39 and activates AMPK-Nrf2 signaling to protect osteoblasts from dexamethasone-induced oxidative injury and cytotoxicity
Abstract
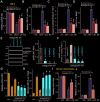
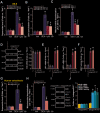
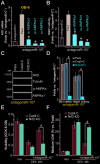
To human osteoblasts dexamethasone (DEX) treatment induces significant oxidative injury and cytotoxicity. Inhibition of CAB39 (calcium binding protein 39)-targeting microRNA can induce CAB39 upregulation, activating AMP-activated protein kinase (AMPK) signaling and offering osteoblast cytoprotection. Here we identified a novel CAB39-targeting miRNA: the microRNA-107 (miR-107). RNA-Pull down assay results demonstrated that the biotinylated-miR-107 directly binds to CAB39 mRNA in OB-6 human osteoblastic cells. Forced overexpression of miR-107, by infection of pre-miR-107 lentivirus or transfection of wild-type miR-107 mimic, largely inhibited CAB39 expression in OB-6 cells and primary human osteoblasts. Contrarily, miR-107 inhibition, by antagomiR-107, increased its expression, resulting in AMPK cascade activation. AntagomiR-107 largely attenuated DEX-induced cell death and apoptosis in OB-6 cells and human osteoblasts. Importantly, osteoblast cytoprotection by antagomiR-107 was abolished with AMPK in-activation by AMPKα1 dominant negative mutation, silencing or knockout. Further studies demonstrated that antagomiR-107 activated AMPK downstream Nrf2 cascade to inhibit DEX-induced oxidative injury. Conversely, Nrf2 knockout almost abolished antagomiR-107-induced osteoblast cytoprotection against DEX. Collectively, miR-107 inhibition induced CAB39 upregulation and activated AMPK-Nrf2 signaling to protect osteoblasts from DEX-induced oxidative injury and cytotoxicity.
Keywords: AMPK-Nrf2 signaling; CAB39; dexamethasone; miR-107; osteoblasts.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

