Sestrin2 inhibits YAP activation and negatively regulates corneal epithelial cell proliferation
- PMID: 32528056
- PMCID: PMC7338388
- DOI: 10.1038/s12276-020-0446-5
Sestrin2 inhibits YAP activation and negatively regulates corneal epithelial cell proliferation
Abstract
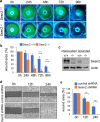
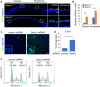
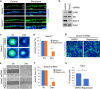
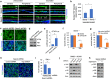
Corneal wound healing is essential for the maintenance of corneal integrity and transparency and involves a series of physiological processes that depend on the proliferation of epithelial cells. However, the molecular mechanisms that control corneal epithelial cell proliferation are poorly understood. Here, we show that Sestrin2, a stress-inducible protein, is downregulated in the corneal epithelium during wound healing and that the proliferation of epithelial basal cells is enhanced in Sestrin2-deficient mice. We also show that YAP, a major downstream effector of the Hippo signaling pathway, regulates cell proliferation during corneal epithelial wound repair and that Sestrin2 suppresses its activity. Moreover, increased levels of reactive oxygen species in the Sestrin2-deficient corneal epithelium promote the nuclear localization and dephosphorylation of YAP, activating it to enhance the proliferation of corneal epithelial cells. These results reveal that Sestrin2 is a negative regulator of YAP, which regulates the proliferative capacity of basal epithelial cells, and may serve as a potential therapeutic target for corneal epithelial damage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
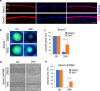
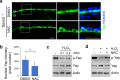
Similar articles
-
An agonist of the adenosine A2A receptor, CGS21680, promotes corneal epithelial wound healing via the YAP signalling pathway.Br J Pharmacol. 2024 Oct;181(19):3779-3795. doi: 10.1111/bph.16468. Epub 2024 Jun 15. Br J Pharmacol. 2024. PMID: 38877785
-
Agrin Promotes Limbal Stem Cell Proliferation and Corneal Wound Healing Through Hippo-Yap Signaling Pathway.Invest Ophthalmol Vis Sci. 2020 May 11;61(5):7. doi: 10.1167/iovs.61.5.7. Invest Ophthalmol Vis Sci. 2020. PMID: 32392315 Free PMC article.
-
Inactivation of Lats1 and Lats2 highlights the role of hippo pathway effector YAP in larynx and vocal fold epithelium morphogenesis.Dev Biol. 2021 May;473:33-49. doi: 10.1016/j.ydbio.2021.01.012. Epub 2021 Jan 28. Dev Biol. 2021. PMID: 33515576 Free PMC article.
-
Study of corneal epithelial progenitor origin and the Yap1 requirement using keratin 12 lineage tracing transgenic mice.Sci Rep. 2016 Oct 13;6:35202. doi: 10.1038/srep35202. Sci Rep. 2016. PMID: 27734924 Free PMC article.
-
YAP regulates the expression of Hoxa1 and Hoxc13 in mouse and human oral and skin epithelial tissues.Mol Cell Biol. 2015 Apr;35(8):1449-61. doi: 10.1128/MCB.00765-14. Epub 2015 Feb 17. Mol Cell Biol. 2015. PMID: 25691658 Free PMC article.
Cited by
-
Sestrin2 in cancer: a foe or a friend?Biomark Res. 2022 May 8;10(1):29. doi: 10.1186/s40364-022-00380-6. Biomark Res. 2022. PMID: 35527284 Free PMC article. Review.
-
Sestrin2 attenuates renal damage by regulating Hippo pathway in diabetic nephropathy.Cell Tissue Res. 2022 Oct;390(1):93-112. doi: 10.1007/s00441-022-03668-z. Epub 2022 Jul 12. Cell Tissue Res. 2022. PMID: 35821438
-
Sestrin2 in diabetes and diabetic complications.Front Endocrinol (Lausanne). 2023 Oct 18;14:1274686. doi: 10.3389/fendo.2023.1274686. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37920252 Free PMC article. Review.
-
The Hippo signalling pathway and its implications in human health and diseases.Signal Transduct Target Ther. 2022 Nov 8;7(1):376. doi: 10.1038/s41392-022-01191-9. Signal Transduct Target Ther. 2022. PMID: 36347846 Free PMC article. Review.
-
Sestrin2 ameliorates age-related spontaneous benign prostatic hyperplasia via activation of AMPK/mTOR dependent autophagy.Biogerontology. 2025 Jan 24;26(1):48. doi: 10.1007/s10522-025-10184-4. Biogerontology. 2025. PMID: 39853471
References
-
- Tseng SCG. Concept and application of limbal stem cells. Eye. 1989;3:141–157. - PubMed
-
- Sun TT, Lavker RM. Corneal epithelial stem cells: past, present, and future. J. Investig. Dermatol. Symp. Proc. 2004;9:202–207. - PubMed
-
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated Sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

