SARS-CoV-2 COVID-19 susceptibility and lung inflammatory storm by smoking and vaping
- PMID: 32528233
- PMCID: PMC7284674
- DOI: 10.1186/s12950-020-00250-8
SARS-CoV-2 COVID-19 susceptibility and lung inflammatory storm by smoking and vaping
Abstract
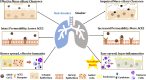
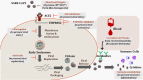
The current pandemic of COVID-19 has caused severe morbidity and mortality across the globe. People with a smoking history have severe disease outcomes by COVID-19 infection. Epidemiological studies show that old age and pre-existing disease conditions (hypertension and diabetes) result in severe disease outcome and mortality amongst COVID-19 patients. Evidences suggest that the S1 domain of the SARS-CoV-2 (causative agent of COVID-19) membrane spike has a high affinity towards the angiotensin-converting enzyme 2 (ACE2) receptor found on the host's lung epithelium. Likewise, TMPRSS2 protease has been shown to be crucial for viral activation thus facilitating the viral engulfment. The viral entry has been shown to cause 'cytokine storm' involving excessive production of pro-inflammatory cytokines/chemokines including IL-6, TNF-α, IFN-γ, IL-2, IL-7, IP-10, MCP-3 or GM-CSF, which is augmented by smoking. Future research could target these inflammatory-immunological responses to develop effective therapy for COVID-19. This mini-review provides a consolidated account on the role of inflammation and immune responses, proteases, and epithelial permeability by smoking and vaping during SARS-CoV2 infection with future directions of research, and provides a list of the potential targets for therapies particularly controlling cytokine storms in the lung.
Keywords: ACE2; COVID-19; E-cigarettes; Inflammation; SARS-CoV2; Smoking; Vaping.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsNone.
Figures
References
-
- Fuk-Woo Chan J, Yuan S, Kok K-H, Kai-Wang To K, Chu H, Yang J, Xing F, Liu J, Chik-Yan Yip C, Wing-Shan Poon R, Tsoi H-W, Kam-Fai Lo S, Chan K-H, Kwok-Man Poon V, Chan W-M, Ip JD, Cai J-P, Chi-Chung Cheng V, Chen H, Kim-Ming Hui C, Yuen K-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–23. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

