Biological Considerations in Scaling Up Therapeutic Cell Manufacturing
- PMID: 32528277
- PMCID: PMC7247829
- DOI: 10.3389/fphar.2020.00654
Biological Considerations in Scaling Up Therapeutic Cell Manufacturing
Abstract
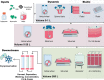
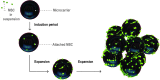
Cell therapeutics - using cells as living drugs - have made advances in many areas of medicine. One of the most clinically studied cell-based therapy products is mesenchymal stromal cells (MSCs), which have shown promising results in promoting tissue regeneration and modulating inflammation. However, MSC therapy requires large numbers of cells, the generation of which is not feasible via conventional planar tissue culture methods. Scale-up manufacturing methods (e.g., propagation on microcarriers in stirred-tank bioreactors), however, are not specifically tailored for MSC expansion. These processes may, in principle, alter the cell secretome, a vital component underlying the immunosuppressive properties and clinical effectiveness of MSCs. This review outlines our current understanding of MSC properties and immunomodulatory function, expansion in commercial manufacturing systems, and gaps in our knowledge that need to be addressed for effective up-scaling commercialization of MSC therapy.
Keywords: biomanufacturing; bioreactors; cell therapy; immunomodulatory; mesenchymal stromal cells; microcarriers; secretome.
Copyright © 2020 Cherian, Bhuvan, Meagher and Heng.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

