Point-Of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-automatic Bioreactor: Experience From an Academic Phase I Clinical Trial
- PMID: 32528460
- PMCID: PMC7259426
- DOI: 10.3389/fimmu.2020.00482
Point-Of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-automatic Bioreactor: Experience From an Academic Phase I Clinical Trial
Abstract
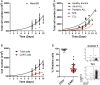
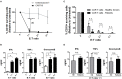
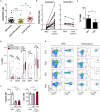
Development of semi-automated devices that can reduce the hands-on time and standardize the production of clinical-grade CAR T-cells, such as CliniMACS Prodigy from Miltenyi, is key to facilitate the development of CAR T-cell therapies, especially in academic institutions. However, the feasibility of manufacturing CAR T-cell products from heavily pre-treated patients with this system has not been demonstrated yet. Here we report and characterize the production of 28 CAR T-cell products in the context of a phase I clinical trial for CD19+ B-cell malignancies (NCT03144583). The system includes CD4-CD8 cell selection, lentiviral transduction and T-cell expansion using IL-7/IL-15. Twenty-seven out of 28 CAR T-cell products manufactured met the full list of specifications and were considered valid products. Ex vivo cell expansion lasted an average of 8.5 days and had a mean transduction rate of 30.6 ± 13.44%. All products obtained presented cytotoxic activity against CD19+ cells and were proficient in the secretion of pro-inflammatory cytokines. Expansion kinetics was slower in patient's cells compared to healthy donor's cells. However, product potency was comparable. CAR T-cell subset phenotype was highly variable among patients and largely determined by the initial product. TCM and TEM were the predominant T-cell phenotypes obtained. 38.7% of CAR T-cells obtained presented a TN or TCM phenotype, in average, which are the subsets capable of establishing a long-lasting T-cell memory in patients. An in-depth analysis to identify individual factors contributing to the optimal T-cell phenotype revealed that ex vivo cell expansion leads to reduced numbers of TN, TSCM, and TEFF cells, while TCM cells increase, both due to cell expansion and CAR-expression. Overall, our results show for the first time that clinical-grade production of CAR T-cells for heavily pre-treated patients using CliniMACS Prodigy system is feasible, and that the obtained products meet the current quality standards of the field. Reduced ex vivo expansion may yield CAR T-cell products with increased persistence in vivo.
Keywords: CAR T-cell production; CD19; CliniMACS Prodigy; chimeric antigen receptor; immunotherapy; leukemia; lymphoma.
Copyright © 2020 Castella, Caballero-Baños, Ortiz-Maldonado, González-Navarro, Suñé, Antoñana-Vidósola, Boronat, Marzal, Millán, Martín-Antonio, Cid, Lozano, García, Tabera, Trias, Perpiña, Canals, Baumann, Benítez-Ribas, Campo, Yagüe, Urbano-Ispizua, Rives, Delgado and Juan.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

