Single-administration, thermostable human papillomavirus vaccines prepared with atomic layer deposition technology
- PMID: 32528733
- PMCID: PMC7265342
- DOI: 10.1038/s41541-020-0195-4
Single-administration, thermostable human papillomavirus vaccines prepared with atomic layer deposition technology
Abstract
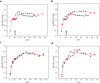
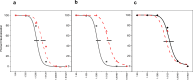
Cold-chain requirements affect worldwide distribution of many vaccines. In addition, vaccines requiring multiple doses impose logistical and financial burdens, as well as patient compliance barriers. To address such limitations, we have developed new technologies to prepare thermostable, single-shot, prime-boost microparticle vaccines. Antigen/adjuvant formulations containing glass-forming polymers and trehalose first are spray-dried to form glassy microparticles that confer thermostability. Atomic layer deposition (ALD) reactions conducted in fluidized beds are then used to coat the microparticles with defined numbers of molecular layers of alumina that modulate the timed release of the internalized antigen and act as adjuvants. We have used a model HPV16 L1 capsomere antigen to evaluate the properties of these technologies. Thermostabilized powders containing HPV16 L1 capsomeres were prepared by spray-drying, coated by ALD with up to 500 molecular layers of alumina, and injected into mice. Antigen distribution was assessed by live-animal IR dye tracking of injected labeled antigen. Antibody responses were measured weekly by ELISA, and neutralizing antibodies were measured by pseudovirus neutralization assays at selected time points. Thermostability was evaluated by measuring antibody responses after incubating ALD-coated antigen powders for one month at 50 °C. Single doses of the ALD-coated vaccine formulations elicited a prime-boost immune response, and produced neutralizing responses and antibody titers that were equivalent or superior to conventional prime-boost doses of liquid formulations. Antibody titers were unaffected by month-long incubation of the formulations at 50 °C. Single-dose, thermostable antigen preparations may overcome current limitations in HPV vaccine delivery as well as being widely applicable to other antigens.
Keywords: Biotechnology; Immunology.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsR.L.G. and T.W.R. have financial interests in VitriVax, Inc., which has licensed patents from the University of Colorado concerning the thermostabilization and atomic layer deposition technologies. The other authors (N.M.M., M.D., H.F., and S.G.) have no competing interests.
Figures



References
-
- Volkin, D. B., Burke, C. J., Sanyal, G. & Middaugh, C. R. Analysis of vaccine stability. Dev. Biol. Stand.87, 135–142 (1996). - PubMed
-
- Chen, D. & Kristensen, D. Opportunities and challenges of developing thermostable vaccines. Expert Rev. Vaccines8, 547–557 (2009). - PubMed
-
- Kumru, O. S. et al. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals42, 237–259 (2014). - PubMed
-
- Kristensen, D. & Chen, D. Stabilization of vaccines: lessons learned. Hum. Vaccin.6, 229–231 (2010). - PubMed
-
- Clausi, A. L., Merkley, S. A., Carpenter, J. F. & Randolph, T. W. Inhibition of aggregation of aluminum hydroxide adjuvant during freezing and drying. J. Pharm. Sci.97, 2051–2061 (2008). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

