Metabolic Plasticity of Melanoma Cells and Their Crosstalk With Tumor Microenvironment
- PMID: 32528879
- PMCID: PMC7256186
- DOI: 10.3389/fonc.2020.00722
Metabolic Plasticity of Melanoma Cells and Their Crosstalk With Tumor Microenvironment
Abstract
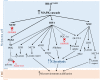
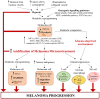
Cutaneous melanoma (CM) is a highly aggressive and drug resistant solid tumor, showing an impressive metabolic plasticity modulated by oncogenic activation. In particular, melanoma cells can generate adenosine triphosphate (ATP) during cancer progression by both cytosolic and mitochondrial compartments, although CM energetic request mostly relies on glycolysis. The upregulation of glycolysis is associated with constitutive activation of BRAF/MAPK signaling sustained by BRAFV600E kinase mutant. In this scenario, the growth and progression of CM are strongly affected by melanoma metabolic changes and interplay with tumor microenvironment (TME) that sustain tumor development and immune escape. Furthermore, CM metabolic plasticity can induce a metabolic adaptive response to BRAF/MEK inhibitors (BRAFi/MEKi), associated with the shift from glycolysis toward oxidative phosphorylation (OXPHOS). Therefore, in this review article we survey the metabolic alterations and plasticity of CM, its crosstalk with TME that regulates melanoma progression, drug resistance and immunosurveillance. Finally, we describe hallmarks of melanoma therapeutic strategies targeting the shift from glycolysis toward OXPHOS.
Keywords: OXPHOS; cutaneous melanoma; metabolic alterations; therapeutic strategies; tumor microenvironment.
Copyright © 2020 Avagliano, Fiume, Pelagalli, Sanità, Ruocco, Montagnani and Arcucci.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

