Antineoplastic Agents Targeting Sphingolipid Pathways
- PMID: 32528896
- PMCID: PMC7256948
- DOI: 10.3389/fonc.2020.00833
Antineoplastic Agents Targeting Sphingolipid Pathways
Abstract
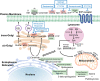
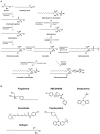
Emerging studies in the enigmatic area of bioactive lipids have made many exciting new discoveries in recent years. Once thought to play a strictly structural role in cellular function, it has since been determined that sphingolipids and their metabolites perform a vast variety of cellular functions beyond what was previously believed. Of utmost importance is their role in cellular signaling, for it is now well understood that select sphingolipids serve as bioactive molecules that play critical roles in both cancer cell death and survival, as well as other cellular responses such as chronic inflammation, protection from intestinal pathogens, and intrinsic protection from intestinal contents, each of which are associated with oncogenesis. Importantly, it has been demonstrated time and time again that many different tumors display dysregulation of sphingolipid metabolism, and the exact profile of said dysregulation has been proven to be useful in determining not only the presence of a tumor, but also the susceptibility to various chemotherapeutic drugs, as well as the metastasizing characteristics of the malignancies. Since these discoveries surfaced it has become apparent that the understanding of sphingolipid metabolism and profile will likely become of great importance in the clinic for both chemotherapy and diagnostics of cancer. The goal of this paper is to provide a comprehensive review of the current state of chemotherapeutic agents that target sphingolipid metabolism that are undergoing clinical trials. Additionally, we will formulate questions involving the use of sphingolipid metabolism as chemotherapeutic targets in need of further research.
Keywords: ceramides; lipid biomarkers; sphingolipids; sphingomyelin; sphingosine-1-phosphate.
Copyright © 2020 Kroll, Cho and Kang.
Figures
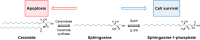
References
Publication types
LinkOut - more resources
Full Text Sources

