Management of adverse events of EUS-directed transgastric ERCP procedure
- PMID: 32529164
- PMCID: PMC7276935
- DOI: 10.1016/j.vgie.2020.02.005
Management of adverse events of EUS-directed transgastric ERCP procedure
Abstract
Background and aims: Accessing the pancreatobiliary region in patients with a history of Roux-en-Y gastric bypass (RYGB) can be challenging. Traditionally, techniques such as percutaneous biliary drainage, enteroscopy-assisted ERCP, and laparoscopy-assisted ERCP have been used. However, each technique has its limitations. EUS-directed transgastric ERCP (EDGE) using a lumen-apposing metal stent (LAMS) has emerged as a novel endoscopic technique for ERCP in patients who have undergone RYGB. The aim of this case series was to highlight LAMS-related shortcomings and adverse events during the periprocedural period.
Methods: This was a retrospective review of 4 patients with RYGB anatomy who underwent EDGE for the management of pancreaticobiliary disease and experienced LAMS-related adverse events. Techniques for managing and avoiding these events are discussed.
Results: Four patients underwent EDGE with both technical and clinical success. Slight LAMS migration with partial mucosal overgrowth was encountered in 1 case and was managed by LAMS removal. A large, bleeding, distal marginal ulcer after the EDGE procedure was encountered in the second case and was managed with proton pump inhibitor and removal of the LAMS, with fistula treatment with argon plasma coagulation used to enhance closure. The third case was complicated by moderate intraprocedural bleeding after LAMS dilation, which was managed by applying balloon tamponade and placing a through-the-scope esophageal stent across the LAMS. Last, preferential food passage to the excluded stomach was noted in the fourth case and resulted in symptomatic distention. The symptomatic distention was managed by another de novo jejunogastrostomy using a LAMS for drainage.
Conclusions: Despite its feasibility and acceptable safety profile, the use of LAMSs during EDGE could be associated with several procedure-specific adverse events, which can be avoided or managed endoscopically with no further consequence.
Keywords: EDGE, EUS-directed transgastric ERCP; LAMS, lumen-apposing metal stent; RYGB, Roux-en-Y gastric bypass.
© 2020 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc.
Figures

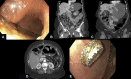
References
-
- Skinner M., Popa D., Neumann H. ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 2014;46:560–572. - PubMed
-
- Schreiner M.A., Chang L., Gluck M. Laparoscopy–assisted versus balloon enteroscopy–assisted ERCP in bariatric post–Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748–756. - PubMed
-
- Abbas A.M., Strong A.T., Diehl D.L. Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc. 2018;87:1031–1039. - PubMed
-
- Banerjee N., Parepally M., Byrne T.K. Systematic review of transgastric ERCP in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis. 2017;13:1236–1242. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

