Advances in nanotechnology-based strategies for the treatments of amyotrophic lateral sclerosis
- PMID: 32529183
- PMCID: PMC7280770
- DOI: 10.1016/j.mtbio.2020.100055
Advances in nanotechnology-based strategies for the treatments of amyotrophic lateral sclerosis
Abstract
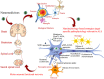
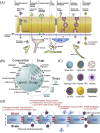
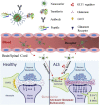
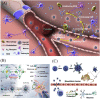
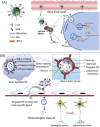
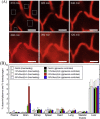
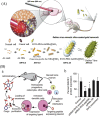
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a progressive neurodegenerative disease that affects both upper and lower motor neurons, which results in loss of muscle control and eventual paralysis [1]. Currently, there are as yet unresolved challenges regarding efficient drug delivery into the central nervous system (CNS). These challenges can be attributed to multiple factors including the presence of the blood-brain barrier (BBB), blood-spinal cord barrier (BSCB), as well as the inherent characteristics of the drugs themselves (e.g. low solubility, insufficient bioavailability/bio-stability, 'off-target' effects) etc. As a result, conventional drug delivery systems may not facilitate adequate dosage of the required drugs for functional recovery in ALS patients. Nanotechnology-based strategies, however, employ engineered nanostructures that show great potential in delivering single or combined therapeutic agents to overcome the biological barriers, enhance interaction with targeted sites, improve drug bioavailability/bio-stability and achieve real-time tracking while minimizing the systemic side-effects. This review provides a concise discussion of recent advances in nanotechnology-based strategies in relation to combating specific pathophysiology relevant to ALS disease progression and investigates the future scope of using nanotechnology to develop innovative treatments for ALS patients.
Keywords: Amyotrophic lateral sclerosis (ALS); Blood-brain barrier; Central nervous system (CNS); Nanotechnology; Neurodegenerative diseases.
© 2020 The Authors.
Figures



References
-
- Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., Van Den Berg L.H. Amyotrophic lateral sclerosis. Nature Rev. Disease Prim. 2017;3:17071. - PubMed
-
- Oskarsson B., Gendron T.F., Staff N.P. Mayo Clinic Proceedings. Elsevier; 2018. Amyotrophic lateral sclerosis: an update for 2018; pp. 1617–1628. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

