HIF-1α is involved in blood-brain barrier dysfunction and paracellular migration of bacteria in pneumococcal meningitis
- PMID: 32529267
- PMCID: PMC7360668
- DOI: 10.1007/s00401-020-02174-2
HIF-1α is involved in blood-brain barrier dysfunction and paracellular migration of bacteria in pneumococcal meningitis
Abstract
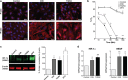
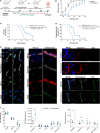
Bacterial meningitis is a deadly disease most commonly caused by Streptococcus pneumoniae, leading to severe neurological sequelae including cerebral edema, seizures, stroke, and mortality when untreated. Meningitis is initiated by the transfer of S. pneumoniae from blood to the brain across the blood-cerebrospinal fluid barrier or the blood-brain barrier (BBB). The underlying mechanisms are still poorly understood. Current treatment strategies include adjuvant dexamethasone for inflammation and cerebral edema, followed by antibiotics. The success of dexamethasone is however inconclusive, necessitating new therapies for controlling edema, the primary reason for neurological complications. Since we have previously shown a general activation of hypoxia inducible factor (HIF-1α) in bacterial infections, we hypothesized that HIF-1α, via induction of vascular endothelial growth factor (VEGF) is involved in transmigration of pathogens across the BBB. In human, murine meningitis brain samples, HIF-1α activation was observed by immunohistochemistry. S. pneumoniae infection in brain endothelial cells (EC) resulted in in vitro upregulation of HIF-1α/VEGF (Western blotting/qRT-PCR) associated with increased paracellular permeability (fluorometry, impedance measurements). This was supported by bacterial localization at cell-cell junctions in vitro and in vivo in brain ECs from mouse and humans (confocal, super-resolution, electron microscopy, live-cell imaging). Hematogenously infected mice showed increased permeability, S. pneumoniae deposition in the brain, along with upregulation of genes in the HIF-1α/VEGF pathway (RNA sequencing of brain microvessels). Inhibition of HIF-1α with echinomycin, siRNA in bEnd5 cells or using primary brain ECs from HIF-1α knock-out mice revealed reduced endothelial permeability and transmigration of S. pneumoniae. Therapeutic rescue using the HIF-1α inhibitor echinomycin resulted in increased survival and improvement of BBB function in S. pneumoniae-infected mice. We thus demonstrate paracellular migration of bacteria across BBB and a critical role for HIF-1α/VEGF therein and hence propose targeting this pathway to prevent BBB dysfunction and ensuing brain damage in infections.
Keywords: Blood–brain barrier (BBB); Dexamethasone; Endothelium; HIF-1α; Meningitis; Paracellular transmigration; Permeability; S. pneumoniae; VEGF.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

