Sacituzumab Govitecan: First Approval
- PMID: 32529410
- PMCID: PMC7288263
- DOI: 10.1007/s40265-020-01337-5
Sacituzumab Govitecan: First Approval
Abstract
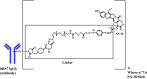
Sacituzumab govitecan (sacituzumab govitecan-hziy; Trodelvy™) is a Trop-2-directed antibody conjugated to a topoisomerase I inhibitor (SN-38) that is being developed by Immunomedics for the treatment of solid tumours, including breast cancer. In April 2020, sacituzumab govitecan received accelerated approval in the USA for the treatment of adult patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for metastatic disease. Sacituzumab govitecan is undergoing phase III development for breast cancer in the USA and EU, and phase II development for urothelial cancer. It is also being explored for brain metastases, glioblastoma, endometrial cancer and prostate cancer. This article summarizes the milestones in the development of sacituzumab govitecan leading to this first approval for mTNBC.
Conflict of interest statement
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Figures
References
-
- Immunomedics. Advanced antibody-based therapeutics [presentation]. 2017. https://www.immunomedics.com. Accessed 20 May 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous