Trends in HIV pre-exposure prophylaxis uptake in Ontario, Canada, and impact of policy changes: a population-based analysis of projected pharmacy data (2015-2018)
- PMID: 32529552
- PMCID: PMC7851246
- DOI: 10.17269/s41997-020-00332-3
Trends in HIV pre-exposure prophylaxis uptake in Ontario, Canada, and impact of policy changes: a population-based analysis of projected pharmacy data (2015-2018)
Abstract
Objectives: HIV pre-exposure prophylaxis (PrEP) is a proven tool for HIV prevention, but PrEP use in Ontario, Canada, and the effects of recent policies are unknown. We estimated the number and characteristics of PrEP users in Ontario and evaluated the impacts of policy changes between July 2015 and June 2018.
Methods: We obtained tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) dispensation data for Ontario from IQVIA, and applied an algorithm to identify use for PrEP. We report prevalent PrEP use for the second quarter of 2018 according to age, sex, region, prescriber specialty, and payer type, and generate "PrEP-to-need ratios" (PNR) by dividing these numbers by the estimated numbers of new HIV diagnoses. We used interventional autoregressive integrated moving average models to examine the impact of three policy changes on PrEP use: Health Canada approval (February 2016), availability of generic TDF/FTC and partial public drug coverage (September 2017), and public drug coverage for individuals aged < 25 years (January 2018).
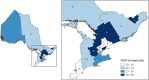
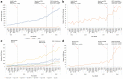
Results: The estimated number of individuals receiving PrEP increased 713%, from 374 in 2015 Q3 to 3041 in 2018 Q2. Among PrEP users in 2018 Q2, 97.5% were male, 60.4% were < 40 years, 67.7% obtained PrEP from a family physician, 77.2% used private insurance, and 67.0% were in Toronto. PNRs were highest in 30-39-year-olds, males, Toronto and the Central East and West regions. Time series analyses found that Health Canada approval (p = 0.0001) and introducing generics/partial public drug coverage (p = 0.002) led to significantly increased use.
Conclusions: PrEP use has risen in Ontario in association with favourable policy changes, but remains far below guideline recommendations.
RéSUMé: OBJECTIFS: La prophylaxie pré-exposition (PPrE) est un outil éprouvé pour prévenir le VIH, mais le recours à la PPrE en Ontario (Canada) et les effets de politiques récentes sont inconnus. Nous avons estimé le nombre et les caractéristiques des utilisateurs de la PPrE en Ontario et évalué les incidences de changements de politique survenus entre juillet 2015 et juin 2018. MéTHODE: Nous avons obtenu auprès d’IQVIA des données sur l’administration de fumarate de ténofovir disoproxil (FTD) et d’emtricitabine (FTC) en Ontario et appliqué un algorithme pour déterminer le recours à la PPrE. Nous présentons la prévalence du recours à la PPrE au deuxième trimestre de 2018 selon l’âge, le sexe, la région, la spécialité du médecin prescripteur et le type de payeur, et nous générons des « ratios PPrE-besoins » (RPB) en divisant ces nombres par les nombres estimatifs de nouveaux diagnostics de VIH. Nous avons utilisé des modèles interventionnels fondés sur la moyenne mobile intégrée autorégressive pour examiner les incidences de trois changements de politique sur le recours à la PPrE : l’approbation par Santé Canada (février 2016); la disponibilité de versions génériques du FTD et de la FTC et leur couverture partielle par le régime public d’assurance-médicaments (septembre 2017); et la couverture des moins de 25 ans par le régime public d’assurance-médicaments (janvier 2018). RéSULTATS: Le nombre estimatif de personnes recevant la PPrE a augmenté de 713 %, passant de 374 au troisième trimestre de 2015 à 3041 au deuxième trimestre de 2018. Chez les utilisateurs de la PPrE au deuxième trimestre de 2018, 97,5 % étaient des hommes, 60,4 % avaient moins de 40 ans, 67,7 % obtenaient la PPrE auprès d’un médecin de famille, 77,2 % utilisaient une assurance privée, et 67,0 % vivaient à Toronto. Les RPB les plus élevés ont été observés chez les 30 à 39 ans, chez les hommes et chez les résidents de Toronto et des régions du Centre-Est et du Centre-Ouest. Selon les résultats d’analyses des séries chronologiques, l’approbation par Santé Canada (p = 0,0001) et l’introduction de versions génériques/la couverture partielle par le régime public d’assurance-médicaments (p = 0,002) ont entraîné des hausses significatives du recours à la PPrE. CONCLUSIONS: Le recours à la PPrE a augmenté en Ontario en lien avec des changements de politique favorables, mais il demeure très en-deçà des recommandations des lignes directrices.
Keywords: HIV; Policy; Pre-exposure prophylaxis; Prevention and control; Public health.
Conflict of interest statement
In the past 3 years, DHST’s institution has received research support for investigator-initiated research studies from Gilead Sciences and Viiv Healthcare, outside the submitted work. DHST is a Site Principal Investigator for clinical trials sponsored by Glaxo Smith Kline, outside the submitted work.
Figures
References
-
- Choopanya, K., Martin, M., Suntharasamai, P., Sangkum, U., Mock, P. A., Leethochawalit, M., et al. (2013). Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London, England), 381(9883), 2083–2090 10.1016/S0140-6736(13)61127-7. - PubMed
-
- Dearing J, Kee K. Historical roots of dissemination and implementation science. In: Brownson RC, Colditz GA, Proctor EK, editors. Dissemination and implementation research in health. New York: Oxford University Press; 2012.
-
- Dinh, T., & Sutherland, G. (2017). Understanding the gap: a Pan-Canadian analysis of prescription drug insurance coverage. Report. Canadian Alliance for Sustainable Health Care. http://innovativemedicines.ca/wp-content/uploads/2017/12/20170712-unders...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

