Efficacy and tolerability of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: a systematic review and individual patient data meta-analysis
- PMID: 32530424
- PMCID: PMC7391007
- DOI: 10.1016/S1473-3099(20)30064-5
Efficacy and tolerability of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: a systematic review and individual patient data meta-analysis
Abstract
Background: Malaria in pregnancy affects both the mother and the fetus. However, evidence supporting treatment guidelines for uncomplicated (including asymptomatic) falciparum malaria in pregnant women is scarce and assessed in varied ways. We did a systematic literature review and individual patient data (IPD) meta-analysis to compare the efficacy and tolerability of different artemisinin-based or quinine-based treatments for malaria in pregnant women.
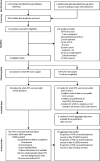
Methods: We did a systematic review of interventional or observational cohort studies assessing the efficacy of artemisinin-based or quinine-based treatments in pregnancy. Seven databases (MEDLINE, Embase, Global Health, Cochrane Library, Scopus, Web of Science, and Literatura Latino Americana em Ciencias da Saude) and two clinical trial registries (International Clinical Trials Registry Platform and ClinicalTrials.gov) were searched. The final search was done on April 26, 2019. Studies that assessed PCR-corrected treatment efficacy in pregnancy with follow-up of 28 days or more were included. Investigators of identified studies were invited to share data from individual patients. The outcomes assessed included PCR-corrected efficacy, PCR-uncorrected efficacy, parasite clearance, fever clearance, gametocyte development, and acute adverse events. One-stage IPD meta-analysis using Cox and logistic regression with random-effects was done to estimate the risk factors associated with PCR-corrected treatment failure, using artemether-lumefantrine as the reference. This study is registered with PROSPERO, CRD42018104013.
Findings: Of the 30 studies assessed, 19 were included, representing 92% of patients in the literature (4968 of 5360 episodes). Risk of PCR-corrected treatment failure was higher for the quinine monotherapy (n=244, adjusted hazard ratio [aHR] 6·11, 95% CI 2·57-14·54, p<0·0001) but lower for artesunate-amodiaquine (n=840, 0·27, 95% 0·14-0·52, p<0·0001), artesunate-mefloquine (n=1028, 0·56, 95% 0·34-0·94, p=0·03), and dihydroartemisinin-piperaquine (n=872, 0·35, 95% CI 0·18-0·68, p=0·002) than artemether-lumefantrine (n=1278) after adjustment for baseline asexual parasitaemia and parity. The risk of gametocyte carriage on day 7 was higher after quinine-based therapy than artemisinin-based treatment (adjusted odds ratio [OR] 7·38, 95% CI 2·29-23·82).
Interpretation: Efficacy and tolerability of artemisinin-based combination therapies (ACTs) in pregnant women are better than quinine. The lower efficacy of artemether-lumefantrine compared with other ACTs might require dose optimisation.
Funding: The Bill & Melinda Gates Foundation, ExxonMobil Foundation, and the University of Oxford Clarendon Fund.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Evidence for treating malaria with artemisinin-based combination therapy in the first trimester of pregnancy.Lancet Infect Dis. 2020 Aug;20(8):880-881. doi: 10.1016/S1473-3099(20)30131-6. Epub 2020 Apr 29. Lancet Infect Dis. 2020. PMID: 32546326 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

