Tumor-associated macrophage-derived inflammatory cytokine enhances malignant potential of malignant pleural mesothelioma
- PMID: 32530527
- PMCID: PMC7419052
- DOI: 10.1111/cas.14523
Tumor-associated macrophage-derived inflammatory cytokine enhances malignant potential of malignant pleural mesothelioma
Abstract
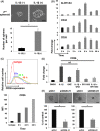
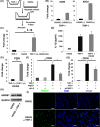
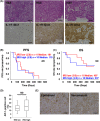
Malignant pleural mesothelioma (MPM) is an asbestos-related aggressive malignant neoplasm. Due to the difficulty of achieving curative surgical resection in most patients with MPM, a combination chemotherapy of cisplatin and pemetrexed has been the only approved regimen proven to improve the prognosis of MPM. However, the median overall survival time is at most 12 mo even with this regimen. There has been therefore a pressing need to develop a novel chemotherapeutic strategy to bring about a better outcome for MPM. We found that expression of interleukin-1 receptor (IL-1R) was upregulated in MPM cells compared with normal mesothelial cells. We also investigated the biological significance of the interaction between pro-inflammatory cytokine IL-1β and the IL-1R in MPM cells. Stimulation by IL-1β promoted MPM cells to form spheroids along with upregulating a cancer stem cell marker CD26. We also identified tumor-associated macrophages (TAMs) as the major source of IL-1β in the MPM microenvironment. Both high mobility group box 1 derived from MPM cells and the asbestos-activated inflammasome in TAMs induced the production of IL-1β, which resulted in enhancement of the malignant potential of MPM. We further performed immunohistochemical analysis using clinical MPM samples obtained from patients who were treated with the combination of platinum plus pemetrexed, and found that the overexpression of IL-1R tended to correlate with poor overall survival. In conclusion, the interaction between MPM cells and TAMs through a IL-1β/IL-1R signal could be a promising candidate as the target for novel treatment of MPM (Hyogo College of Medicine clinical trial registration number: 2973).
Keywords: IL-1β; asbestos; inflammasome; malignant pleural mesothelioma; tumor-associated macrophage.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Scherpereel A, Wallyn F, Albelda SM, Munck C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19(3):e161‐e172. - PubMed
-
- Quispel‐Janssen J, van der Noort V, de Vries JF, et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. 2018;13(10):1569‐1576. - PubMed
-
- Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT‐1501 MAPS2): a multicentre, open‐label, randomised, non‐comparative, phase 2 trial. Lancet Oncol. 2019;20(2):239‐253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

