Lipid and protein dynamics that shape nuclear envelope identity
- PMID: 32530796
- PMCID: PMC7353140
- DOI: 10.1091/mbc.E18-10-0636
Lipid and protein dynamics that shape nuclear envelope identity
Abstract
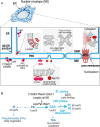
The nuclear envelope (NE) is continuous with the endoplasmic reticulum (ER), yet the NE carries out many functions distinct from those of bulk ER. This functional specialization depends on a unique protein composition that defines NE identity and must be both established and actively maintained. The NE undergoes extensive remodeling in interphase and mitosis, so mechanisms that seal NE holes and protect its unique composition are critical for maintaining its functions. New evidence shows that closure of NE holes relies on regulated de novo lipid synthesis, providing a link between lipid metabolism and generating and maintaining NE identity. Here, we review regulation of the lipid bilayers of the NE and suggest ways to generate lipid asymmetry across the NE despite its direct continuity with the ER. We also discuss the elusive mechanism of membrane fusion during nuclear pore complex (NPC) biogenesis. We propose a model in which NPC biogenesis is carefully controlled to ensure that a permeability barrier has been established before membrane fusion, thereby avoiding a major threat to compartmentalization.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

