Ion channel formation by N-terminally truncated Aβ (4-42): relevance for the pathogenesis of Alzheimer's disease
- PMID: 32531337
- PMCID: PMC8132454
- DOI: 10.1016/j.nano.2020.102235
Ion channel formation by N-terminally truncated Aβ (4-42): relevance for the pathogenesis of Alzheimer's disease
Abstract
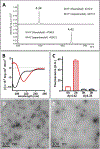
Aβ deposition is a pathological hallmark of Alzheimer's disease (AD). Besides the full-length amyloid forming peptides (Aβ1-40 and Aβ1-42), biochemical analyses of brain deposits have identified a variety of N- and C-terminally truncated Aβ variants in sporadic and familial AD patients. However, their relevance for AD pathogenesis remains largely understudied. We demonstrate that Aβ4-42 exhibits a high tendency to form β-sheet structures leading to fast self-aggregation and formation of oligomeric assemblies. Atomic force microscopy and electrophysiological studies reveal that Aβ4-42 forms highly stable ion channels in lipid membranes. These channels that are blocked by monoclonal antibodies specifically recognizing the N-terminus of Aβ4-42. An Aβ variant with a double truncation at phenylalanine-4 and leucine 34, (Aβ4-34), exhibits unstable channel formation capability. Taken together the results presented herein highlight the potential benefit of C-terminal proteolytic cleavage and further support an important pathogenic role for N-truncated Aβ species in AD pathophysiology.
Keywords: Amyloid; Electrophysiology; Oligomers; Truncated Aβ peptides.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
N-terminally truncated Aβ4-x proteoforms and their relevance for Alzheimer's pathophysiology.Transl Neurodegener. 2022 Jun 1;11(1):30. doi: 10.1186/s40035-022-00303-3. Transl Neurodegener. 2022. PMID: 35641972 Free PMC article.
-
The metalloprotease ADAMTS4 generates N-truncated Aβ4-x species and marks oligodendrocytes as a source of amyloidogenic peptides in Alzheimer's disease.Acta Neuropathol. 2019 Feb;137(2):239-257. doi: 10.1007/s00401-018-1929-5. Epub 2018 Nov 13. Acta Neuropathol. 2019. PMID: 30426203
-
N-truncated Abeta starting with position four: early intraneuronal accumulation and rescue of toxicity using NT4X-167, a novel monoclonal antibody.Acta Neuropathol Commun. 2013 Sep 6;1:56. doi: 10.1186/2051-5960-1-56. Acta Neuropathol Commun. 2013. PMID: 24252153 Free PMC article.
-
N-Terminally Truncated Aβ Peptide Variants in Alzheimer’s Disease.In: Wisniewski T, editor. Alzheimer’s Disease [Internet]. Brisbane (AU): Codon Publications; 2019 Dec 20. Chapter 7. In: Wisniewski T, editor. Alzheimer’s Disease [Internet]. Brisbane (AU): Codon Publications; 2019 Dec 20. Chapter 7. PMID: 31895514 Free Books & Documents. Review.
-
Emerging roles of N- and C-terminally truncated Aβ species in Alzheimer's disease.Expert Opin Ther Targets. 2019 Dec;23(12):991-1004. doi: 10.1080/14728222.2019.1702972. Epub 2019 Dec 12. Expert Opin Ther Targets. 2019. PMID: 31814468 Review.
Cited by
-
Towards the Integrative Theory of Alzheimer's Disease: Linking Molecular Mechanisms of Neurotoxicity, Beta-amyloid Biomarkers, and the Diagnosis.Curr Alzheimer Res. 2023;20(6):440-452. doi: 10.2174/1567205020666230821141745. Curr Alzheimer Res. 2023. PMID: 37605411 Free PMC article.
-
The structure of tyrosine-10 favors ionic conductance of Alzheimer's disease-associated full-length amyloid-β channels.Nat Commun. 2024 Feb 13;15(1):1296. doi: 10.1038/s41467-023-43821-y. Nat Commun. 2024. PMID: 38351257 Free PMC article.
-
Functionally distinct pericyte subsets differently regulate amyloid-β deposition in patients with Alzheimer's disease.Brain Pathol. 2025 Mar;35(2):e13282. doi: 10.1111/bpa.13282. Epub 2024 Jun 27. Brain Pathol. 2025. PMID: 38932696 Free PMC article.
-
Patients with Alzheimer's disease have an increased removal rate of soluble beta-amyloid-42.PLoS One. 2022 Oct 31;17(10):e0276933. doi: 10.1371/journal.pone.0276933. eCollection 2022. PLoS One. 2022. PMID: 36315527 Free PMC article.
-
N-terminally truncated Aβ4-x proteoforms and their relevance for Alzheimer's pathophysiology.Transl Neurodegener. 2022 Jun 1;11(1):30. doi: 10.1186/s40035-022-00303-3. Transl Neurodegener. 2022. PMID: 35641972 Free PMC article.
References
-
- Laurijssens B, Aujard F, Rahman A. Animal models of Alzheimer’s disease and drug development. Drug Discovery Today: Technologies. 10(3), e319–e327 (2013). - PubMed
-
- Portelius E, Westman-Brinkmalm A, Zetterberg H, Blennow K. Determination of β-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. Journal of proteome research. 5(4), 1010–1016 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

