Low-Fouling Substrates for Plasmonic Sensing of Circulating Biomarkers in Biological Fluids
- PMID: 32531908
- PMCID: PMC7345924
- DOI: 10.3390/bios10060063
Low-Fouling Substrates for Plasmonic Sensing of Circulating Biomarkers in Biological Fluids
Abstract
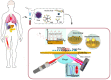
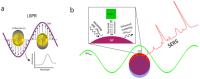
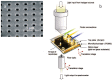
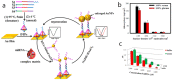
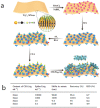
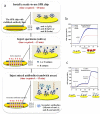
The monitoring of biomarkers in body fluids provides valuable prognostic information regarding disease onset and progression. Most biosensing approaches use noninvasive screening tools and are conducted in order to improve early clinical diagnosis. However, biofouling of the sensing surface may disturb the quantification of circulating biomarkers in complex biological fluids. Thus, there is a great need for antifouling interfaces to be designed in order to reduce nonspecific adsorption and prevent inactivation of biological receptors and loss of sensitivity. To address these limitations and enable their application in clinical practice, a variety of plasmonic platforms have been recently developed for biomarker analysis in easily accessible biological fluids. This review presents an overview of the latest advances in the design of antifouling strategies for the detection of clinically relevant biomarkers on the basis of the characteristics of biological samples. The impact of nanoplasmonic biosensors as point-of-care devices has been examined for a wide range of biomarkers associated with cancer, inflammatory, infectious and neurodegenerative diseases. Clinical applications in readily obtainable biofluids such as blood, saliva, urine, tears and cerebrospinal and synovial fluids, covering almost the whole range of plasmonic applications, from surface plasmon resonance (SPR) to surface-enhanced Raman scattering (SERS), are also discussed.
Keywords: LSPR; SERS; SPR; biological fluids; circulating biomarkers; low-fouling; nanoplasmonics.
Conflict of interest statement
The author declares no conflict of interest.
Figures



Similar articles
-
Enabling Multiplexed Electrochemical Detection of Biomarkers with High Sensitivity in Complex Biological Samples.Acc Chem Res. 2021 Sep 21;54(18):3529-3539. doi: 10.1021/acs.accounts.1c00382. Epub 2021 Sep 3. Acc Chem Res. 2021. PMID: 34478255
-
Advances in nanoplasmonic biosensors for clinical applications.Analyst. 2019 Dec 2;144(24):7105-7129. doi: 10.1039/c9an00701f. Analyst. 2019. PMID: 31663527 Review.
-
Raman, Infrared and Brillouin Spectroscopies of Biofluids for Medical Diagnostics and for Detection of Biomarkers.Crit Rev Anal Chem. 2023;53(7):1561-1590. doi: 10.1080/10408347.2022.2036941. Epub 2022 Feb 14. Crit Rev Anal Chem. 2023. PMID: 35157535 Review.
-
Nanoplasmonic sensors for detecting circulating cancer biomarkers.Adv Drug Deliv Rev. 2018 Feb 1;125:48-77. doi: 10.1016/j.addr.2017.12.004. Epub 2017 Dec 14. Adv Drug Deliv Rev. 2018. PMID: 29247763 Review.
-
Plasmonic-based platforms for diagnosis of infectious diseases at the point-of-care.Biotechnol Adv. 2019 Dec;37(8):107440. doi: 10.1016/j.biotechadv.2019.107440. Epub 2019 Aug 30. Biotechnol Adv. 2019. PMID: 31476421 Review.
Cited by
-
Conducting Polymer-Infused Electrospun Fibre Mat Modified by POEGMA Brushes as Antifouling Biointerface.Biosensors (Basel). 2022 Dec 7;12(12):1143. doi: 10.3390/bios12121143. Biosensors (Basel). 2022. PMID: 36551110 Free PMC article.
-
Surface Plasmon Resonance Biosensors with Magnetic Sandwich Hybrids for Signal Amplification.Biosensors (Basel). 2022 Jul 22;12(8):554. doi: 10.3390/bios12080554. Biosensors (Basel). 2022. PMID: 35892451 Free PMC article.
-
Design of Polymeric Surfaces as Platforms for Streamlined Cancer Diagnostics in Liquid Biopsies.Biosensors (Basel). 2023 Mar 18;13(3):400. doi: 10.3390/bios13030400. Biosensors (Basel). 2023. PMID: 36979612 Free PMC article. Review.
-
Advances in breast cancer diagnosis: a comprehensive review of imaging, biosensors, and emerging wearable technologies.Front Oncol. 2025 Jun 18;15:1587517. doi: 10.3389/fonc.2025.1587517. eCollection 2025. Front Oncol. 2025. PMID: 40606998 Free PMC article. Review.
-
Graphene-Based Electrochemical Biosensors for Breast Cancer Detection.Biosensors (Basel). 2023 Jan 3;13(1):80. doi: 10.3390/bios13010080. Biosensors (Basel). 2023. PMID: 36671915 Free PMC article. Review.
References
-
- The BEST Resource: Harmonizing Biomarker Terminology. [(accessed on 1 April 2020)]; Available online: https://www.fda.gov/media/99221/download.
-
- Veenstra T. Proteomic Applications in Cancer Detection and Discovery. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2013. The search for biomarkers in biofluids; pp. 171–194.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

