Investigating the Potential of Transmucosal Delivery of Febuxostat from Oral Lyophilized Tablets Loaded with a Self-Nanoemulsifying Delivery System
- PMID: 32531910
- PMCID: PMC7356236
- DOI: 10.3390/pharmaceutics12060534
Investigating the Potential of Transmucosal Delivery of Febuxostat from Oral Lyophilized Tablets Loaded with a Self-Nanoemulsifying Delivery System
Abstract
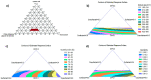
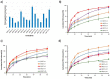
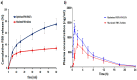
Gout is the most familiar inflammatory arthritis condition caused by the elevation of uric acid in the bloodstream. Febuxostat (FBX) is the latest drug approved by the United States Food and Drug Administration (US FDA) for the treatment of gout and hyperuricemia. FBX is characterized by low solubility resulting in poor gastrointestinal bioavailability. This study aimed at improving the oral bioavailability of FBX by its incorporation into self-nanoemulsifying delivery systems (SNEDS) with minimum globule size and maximum stability index. The SNEDS-incorporated FBX was loaded into a carrier substrate with a large surface area and lyophilized with other excipients to produce a fluffy, porous-like structure tablet for the transmucosal delivery of FBX. The solubility of FBX was studied in various oils, surfactants, and cosurfactants. Extreme vertices design was utilized to optimize FBX-SNEDS, and subsequently loaded into lyophilized tablets along with suitable excipients. The percentages of the main tablet excipients were optimized using a Box-Behnken design to develop self-nanoemulsifying lyophilized tablets (SNELTs) with minimum disintegration time and maximum drug release. The pharmacokinetics parameters of the optimized FBX-SNELTs were tested in healthy human volunteers in comparison with the marketed FBX tablets. The results revealed that the optimized FBX-SNELTs increased the maximum plasma concentration (Cmax) and decreased the time to reach Cmax (Tmax) with a large area under the curve (AUC) as a result of the enhanced relative oral bioavailability of 146.4%. The significant enhancement of FBX bioavailability is expected to lead to reduced side effects and frequency of administration during the treatment of gout.
Keywords: bioavailability; febuxostat; gout; lyophilized tablets; self-nanoemulsifying delivery system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Khanna D., Fitzgerald J.D., Khanna P.P., Bae S., Singh M.K., Neogi T., Pillinger M.H., Merill J., Lee S., Prakash S., et al. American college of rheumatology guidelines for management of gout. part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431–1446. doi: 10.1002/acr.21772. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

