Cerebellar Blood Flow and Gene Expression in Crossed Cerebellar Diaschisis after Transient Middle Cerebral Artery Occlusion in Rats
- PMID: 32531947
- PMCID: PMC7312675
- DOI: 10.3390/ijms21114137
Cerebellar Blood Flow and Gene Expression in Crossed Cerebellar Diaschisis after Transient Middle Cerebral Artery Occlusion in Rats
Abstract
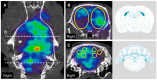
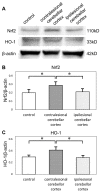
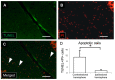
Crossed cerebellar diaschisis (CCD) is a state of hypoperfusion and hypometabolism in the contralesional cerebellar hemisphere caused by a supratentorial lesion, but its pathophysiology is not fully understood. We evaluated chronological changes in cerebellar blood flow (CbBF) and gene expressions in the cerebellum using a rat model of transient middle cerebral artery occlusion (MCAO). CbBF was analyzed at two and seven days after MCAO using single photon emission computed tomography (SPECT). DNA microarray analysis and western blotting of the cerebellar cortex were performed and apoptotic cells in the cerebellar cortex were stained. CbBF in the contralesional hemisphere was significantly decreased and this lateral imbalance recovered over one week. Gene set enrichment analysis revealed that a gene set for "oxidative phosphorylation" was significantly upregulated while fourteen other gene sets including "apoptosis", "hypoxia" and "reactive oxygen species" showed a tendency toward upregulation in the contralesional cerebellum. MCAO upregulated the expressions of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) in the contralesional cerebellar cortex. The number of apoptotic cells increased in the molecular layer of the contralesional cerebellum. Focal cerebral ischemia in our rat MCAO model caused CCD along with enhanced expression of genes related to oxidative stress and apoptosis.
Keywords: apoptosis; cerebral blood flow; crossed cerebellar diaschisis; ischemic stroke; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Baron J.C., Bousser M.G., Comar D., Castaigne P. “Crossed cerebellar diaschisis” in human supratentorial brain infarction. Trans. Am. Neurol. Assoc. 1981;105:459–461. - PubMed
-
- Lim J.S., Ryu Y.H., Kim B.M., Lee J.D. Crossed cerebellar diaschisis due to intracranial hematoma in basal ganglia or thalamus. J. Nucl. Med. 1998;39:2044–2047. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

