Theoretical Insight into the Interaction between Chloramphenicol and Functional Monomer (Methacrylic Acid) in Molecularly Imprinted Polymers
- PMID: 32532004
- PMCID: PMC7312358
- DOI: 10.3390/ijms21114139
Theoretical Insight into the Interaction between Chloramphenicol and Functional Monomer (Methacrylic Acid) in Molecularly Imprinted Polymers
Abstract
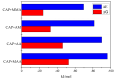
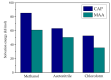
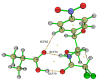
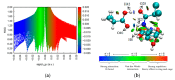
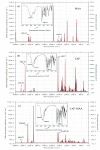
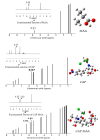
Molecular imprinting technology is a promising method for detecting chloramphenicol (CAP), a broad-spectrum antibiotic with potential toxicity to humans, in animal-derived foods. This work aimed to investigate the interactions between the CAP as a template and functional monomers required for synthesizing efficient molecularly imprinted polymers for recognition and isolation of CAP based on density functional theory. The most suitable monomer, methacrylic acid (MAA), was determined based on interaction energies and Gibbs free energy changes. Further, the reaction sites of CAP and MAA was predicted through the frontier molecular orbitals and molecular electrostatic potentials. Atoms in molecules topology analysis and non-covalent interactions reduced density gradient were applied to investigate different types of non-covalent and inter-atomic interactions. The simulation results showed that CAP was the main electron donor, while MAA was the main electron acceptor. Moreover, the CAP-MAA complex simultaneously involved N-H···O and C=O···H double hydrogen bonds, where the strength of the latter was greater than that of the former. The existence of hydrogen bonds was also confirmed by theoretical and experimental hydrogen nuclear magnetic resonance and Fourier transform infrared spectroscopic analyses. This research can act as an important reference for intermolecular interactions and provide strong theoretical guidance regarding CAP in the synthesis of molecularly imprinted polymers.
Keywords: chloramphenicol; density functional theory; intermolecular interaction; methacrylic acid; molecularly imprinted polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chen H., Ying J., Chen H., Huang J.L., Liao L. LC determination of chloramphenicol in honey using dispersive liquid-liquid microextraction. Chromatographia. 2008;68:629–634. doi: 10.1365/s10337-008-0753-9. - DOI
-
- Zhang R.L., Zhou Z.P., Xie A., Dai J.D., Cui J.Y., Lang J.H., Wei M.B., Dai X.H., Li C.X., Yan Y.S. Preparation of hierarchical porous carbons from sodium carboxymethyl cellulose via halloysite template strategy coupled with KOH-activation for efficient removal of chloramphenicol. J. Taiwan Inst. Chem. Eng. 2017;80:424–433. doi: 10.1016/j.jtice.2017.07.032. - DOI
-
- Qin L., Zhou Z.P., Dai J.D., Ma P., Zhao H.B., He J.S., Xie A., Li C.X., Yan Y.S. Novel N-doped hierarchically porous carbons derived from sustainable shrimp shell for high-performance removal of sulfamethazine and chloramphenicol. J. Taiwan Inst. Chem. Eng. 2016;62:228–238. doi: 10.1016/j.jtice.2016.02.009. - DOI
-
- Yusof N.A., Rahman S.K.A., Hussein M.Z., Ibrahim N.A. Preparation and characterization of molecularly imprinted polymer as SPE sorbent for melamine isolation. Polymers. 2013;5:1215–1228. doi: 10.3390/polym5041215. - DOI
-
- Douny C., Widart J., Pauw d.E., Maghuin-Rogister G., Scippo M.L. Determination of chloramphenicol in honey, shrimp, and poultry meat with liquid chromatography–mass spectrometry: Validation of the method according to commission decision 2002/657/EC. Food Anal. Methods. 2013;6:1458–1465. doi: 10.1007/s12161-013-9596-6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

