The Ubiquitin-Proteasome System Does Not Regulate the Degradation of Porcine β-Microseminoprotein during Sperm Capacitation
- PMID: 32532042
- PMCID: PMC7312034
- DOI: 10.3390/ijms21114151
The Ubiquitin-Proteasome System Does Not Regulate the Degradation of Porcine β-Microseminoprotein during Sperm Capacitation
Abstract
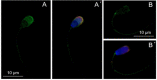
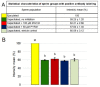
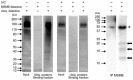
Sperm capacitation, one of the key events during successful fertilization, is associated with extensive structural and functional sperm remodeling, beginning with the modification of protein composition within the sperm plasma membrane. The ubiquitin-proteasome system (UPS), a multiprotein complex responsible for protein degradation and turnover, participates in capacitation events. Previous studies showed that capacitation-induced shedding of the seminal plasma proteins such as SPINK2, AQN1, and DQH from the sperm surface is regulated by UPS. Alterations in the sperm surface protein composition also relate to the porcine β-microseminoprotein (MSMB/PSP94), seminal plasma protein known as immunoglobulin-binding factor, and motility inhibitor. MSMB was detected in the acrosomal region as well as the flagellum of ejaculated boar spermatozoa, while the signal disappeared from the acrosomal region after in vitro capacitation (IVC). The involvement of UPS in the MSMB degradation during sperm IVC was studied using proteasomal interference and ubiquitin-activating enzyme (E1) inhibiting conditions by image-based flow cytometry and Western blot detection. Our results showed no accumulation of porcine MSMB either under proteasomal inhibition or under E1 inhibiting conditions. In addition, the immunoprecipitation study did not detect any ubiquitination of sperm MSMB nor was MSMB detected in the affinity-purified fraction containing ubiquitinated sperm proteins. Based on our results, we conclude that UPS does not appear to be the regulatory mechanism in the case of MSMB and opening new questions for further studies. Thus, the capacitation-induced processing of seminal plasma proteins on the sperm surface may be more complex than previously thought, employing multiple proteolytic systems in a non-redundant manner.
Keywords: MSMB; PSP94; boar; capacitation; spermatozoa; ubiquitin-proteasome system; β-microseminoprotein.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Yanagimachi R. Mammalian fertilization. In: Knobil E., Neill J.D., editors. The Physiology of Reproduction. 2nd ed. Volume 1. Raven Press; New York, NY, USA: 1994. pp. 189–317.
-
- Jonáková V., Ticha M. Boar Seminal Plasma Proteins and Their Binding Properties. A Review. Collect. Czechoslov. Chem. Commun. 2004;69:461–475. doi: 10.1135/cccc20040461. - DOI
-
- Jonáková V., Jonak J., Ticha M. Proteomics of Male Seminal Plasma. In: Jiang Z., Ott T.L., editors. Reproductive Genomics in Domestic Animals. 1st ed. Blackwell Publishing; Oxford, UK: 2010. pp. 339–366.
MeSH terms
Substances
Grants and funding
- GA-18-11275S/Grantová Agentura České Republiky
- 2015-67015-23231/USDA National Institute of Food and Agriculture, Agriculture and Food Research Initiative Competitive Grant
- SV18-08-21230/Internal Grant Agency of Czech University of Life Sciences in Prague
- CZ.1.05/1.1.00/02.0109/BIOCEV from ERDF
- RVO: 86652036/Institute of Biotechnology
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

