Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy
- PMID: 32532087
- PMCID: PMC7352175
- DOI: 10.3390/nu12061742
Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy
Abstract
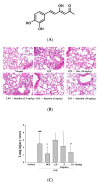
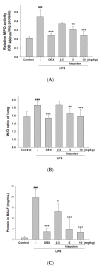
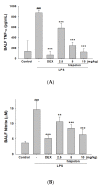
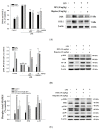
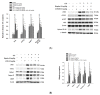
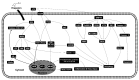
The anti-inflammatory effect of hispolon has identified it as one of the most important compounds from Sanghuangporus sanghuang. The research objectives were to study this compound using an animal model by lipopolysaccharide (LPS)-induced acute lung injury. Hispolon treatment reduced the production of the pro-inflammatory mediator NO, TNF-α, IL-1β, and IL-6 induced by LPS challenge in the lung tissues, as well as decreasing their histological alterations and protein content. Total cell number was also reduced in the bronchoalveolar lavage fluid (BALF). Moreover, hispolon inhibited iNOS, COX-2 and IκB-α and phosphorylated IKK and MAPK, while increasing catalase, SOD, GPx, TLR4, AKT, HO-1, Nrf-2, Keap1 and PPARγ expression, after LPS challenge. It also regulated apoptosis, ER stress and the autophagy signal transduction pathway. The results of this study show that hispolon regulates LPS-induced ER stress (increasing CHOP, PERK, IRE1, ATF6 and GRP78 protein expression), apoptosis (decreasing caspase-3 and Bax and increasing Bcl-2 expression) and autophagy (reducing LC3 I/II and Beclin-1 expression). This in vivo experimental study suggests that hispolon suppresses the LPS-induced activation of inflammatory pathways, oxidative injury, ER stress, apoptosis and autophagy and has the potential to be used therapeutically in major anterior segment lung diseases.
Keywords: ER stress; HO-1; LPS; Nrf-2; anti-inflammation; apoptosis; hispolon.
Conflict of interest statement
All authors have no conflicts of interests exists.
Figures






References
-
- Park J., Chen Y., Zheng M., Ryu J., Cho G.J., Surh Y.J., Sato D., Hamada H., Ryter S.W., Kim U.H., et al. Pterostilbene 4′-β-glucoside attenuates LPS-induced acute lung injury via induction of heme oxygenase-1. Oxid. Med. Cell. Longev. 2018;2018:2747018. doi: 10.1155/2018/2747018. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

