A phase IIb, randomised, parallel-group study: the efficacy, safety and tolerability of once-daily umeclidinium in patients with asthma receiving inhaled corticosteroids
- PMID: 32532275
- PMCID: PMC7291639
- DOI: 10.1186/s12931-020-01400-5
A phase IIb, randomised, parallel-group study: the efficacy, safety and tolerability of once-daily umeclidinium in patients with asthma receiving inhaled corticosteroids
Abstract
Background: Patients with asthma uncontrolled on inhaled corticosteroids may benefit from umeclidinium (UMEC), a long-acting muscarinic antagonist.
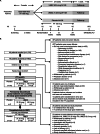
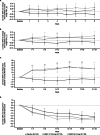
Methods: This Phase IIb, double-blind study included patients with reversible, uncontrolled/partially-controlled asthma for ≥6 months, receiving ≥100 mcg/day fluticasone propionate (or equivalent) for ≥12 weeks. Following a 2-week run-in on open-label fluticasone furoate (FF) 100 mcg, patients were randomised (1:1:1) to receive UMEC 31.25 mcg, UMEC 62.5 mcg or placebo on top of FF 100 mcg once-daily for 24 weeks. As-needed salbutamol was provided. Primary and secondary endpoints were change from baseline in clinic trough forced expiratory volume in 1 s (FEV1) and clinic FEV1 3 h post-dose, respectively, at Week 24. Other endpoints included change from baseline in home daily spirometry (trough FEV1, evening FEV1, morning [pre-dose] and evening peak expiratory flow) over 24 weeks. Safety was assessed throughout the study.
Results: The intent-to-treat population comprised 421 patients (UMEC 31.25 mcg: n =139, UMEC 62.5 mcg: n =139, placebo: n =143). UMEC 31.25 mcg and 62.5 mcg demonstrated significantly greater improvements from baseline in clinic trough FEV1 at Week 24 (difference [95% CI]: 0.176 L [0.092, 0.260; p<0.001] and 0.184 L [0.101, 0.268; p<0.001], respectively), clinic FEV1 3 h post-dose at Week 24 (0.190 L [0.100, 0.279; p<0.001] and 0.198 L [0.109, 0.287; p<0.001], respectively) and mean change from baseline in daily home spirometry over 24 weeks versus placebo. No new safety signals were identified.
Conclusions: UMEC is a highly effective bronchodilator that leads to improved lung function when administered as a single bronchodilator on top of FF in subjects with fully reversible, uncontrolled/partially-controlled moderate asthma. These data support a favourable benefit/risk profile for UMEC (31.25 mcg and 62.5 mcg).
Trial registration: GSK study ID: 205832; Clinicaltrials.gov ID: NCT03012061.
Keywords: Asthma; Forced expiratory volume in 1 s; Inhaled corticosteroid; Long-acting muscarinic antagonist; Umeclidinium.
Conflict of interest statement
The authors met the criteria for authorship as recommended by the International Committee of Medical Journal Editors. ZB, RvM, KR and AF are employees of GSK and own stock in GSK. SP, RD and LL were employees of GSK at the time of study and own stocks in GSK. EK is an employee of Crisor LLC Research and has served on advisory boards, speaker panels or received travel reimbursement from Amphastar, AstraZeneca, Boehringer Ingelheim, Forest, GSK, Mylan, Novartis, Pearl, Sunovion, Teva and Theravance. EK has conducted multicentre clinical research trials for approximately 40 pharmaceutical companies. RN was an employee of Asthma & Allergy Associates at the time of the study, and receives grant funding or research support from Aimmune, AstraZeneca, Attenua, Biocryst, Chiesi, Dyax, Genentech, GSK, Lupin, Menlo, Novartis, Pearl, Pfizer, Sanofi Aventis, Shire, Spirosure, Teva, 3M and Watson. RN is also a consultant or scientific advisor for Boehringer Ingelheim, GSK, Optinose, Stallergenes Greer and CSL Behring and receives speaker’s fees from Boehringer Ingelheim, CSL Behring, GSK, Stallergenes Greer and Merck. DB is an employee of the University of Cincinnati College of Medicine and Bernstein Clinical Research Center. DB receives grant/research/clinical trial support from GSK, Teva, AstraZeneca, Pearl, Novartis, Genentech, Lupin, Merck, Mylan, Boehringer Ingelheim, Amgen, Aimmune, Menlo, Shire, Biocryst and provides consultancy or contributes towards advisory boards for GSK, ALK America, Gerson-Lehman and Guidepoint Global. No authors were paid for the development of the manuscript. ELLIPTA is owned by or licensed to the GSK group of companies.
Figures

References
-
- GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2019. Available from: www.ginasthma.org. Accessed 6 Jan 2020.
-
- National Institutes of Health (NIH) NH, Lung and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma 2007. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines. Accessed 8 Feb 2019.
-
- Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104(40):15858–15863. doi: 10.1073/pnas.0707413104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

