Flow Augmentation in the Myocardium by Ultrasound Cavitation of Microbubbles: Role of Shear-Mediated Purinergic Signaling
- PMID: 32532642
- PMCID: PMC7416478
- DOI: 10.1016/j.echo.2020.03.016
Flow Augmentation in the Myocardium by Ultrasound Cavitation of Microbubbles: Role of Shear-Mediated Purinergic Signaling
Abstract
Background: Ultrasound-mediated cavitation of microbubble contrast agents produces high intravascular shear. We hypothesized that microbubble cavitation increases myocardial microvascular perfusion through shear-dependent purinergic pathways downstream from ATP release that is immediate and sustained through cellular ATP channels such as Pannexin-1.
Methods: Quantitative myocardial contrast echocardiography perfusion imaging and in vivo optical imaging of ATP was performed in wild-type and Pannexin-1-deficient (Panx1-/-) mice before and 5 and 30 minutes after 10 minutes of ultrasound-mediated (1.3 MHz, mechanical index 1.3) myocardial microbubble cavitation. Flow augmentation in a preclinical model closer to humans was evaluated in rhesus macaques undergoing myocardial contrast echocardiography perfusion imaging after high-power cavitation in the apical four-chamber plane for 10 minutes.
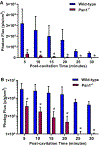
Results: Microbubble cavitation in wild-type mice (n = 7) increased myocardial perfusion by 64% ± 25% at 5 minutes and 95% ± 55% at 30 minutes compared with baseline (P < .05). In Panx1-/- mice (n = 5), perfusion increased by 28% ± 26% at 5 minutes (P = .04) but returned to baseline at 30 minutes. Myocardial ATP signal in wild-type (n = 7) mice undergoing cavitation compared with sham-treated controls (n = 3) was 450-fold higher at 5 minutes and 90-fold higher at 30 minutes after cavitation (P < .001). The ATP signal in Panx1-/- mice (n = 4) was consistently 10-fold lower than that in wild-type mice and was similar to sham controls at 30 minutes. In macaques (n = 8), myocardial perfusion increased twofold in the cavitation-exposed four-chamber plane, similar in degree to that produced by adenosine, but did not increase in the control two-chamber plane.
Conclusions: Cavitation of microbubbles in the myocardial microcirculation produces an immediate release of ATP, likely from cell microporation, as well as sustained release, which is channel dependent and responsible for persistent flow augmentation. These findings provide mechanistic insight by which cavitation improves perfusion and reduces infarct size in patients with myocardial infarction.
Keywords: Cavitation; Contrast ultrasound; Microbubbles; Myocardial blood flow.
Copyright © 2020 American Society of Echocardiography. Published by Elsevier Inc. All rights reserved.
Figures
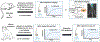


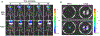


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

